Glioblastoma in a Male with DownÃÆâÃâââ¬Ãâââ¢s Syndrome: A Case Report
Ishita Pant
Pant I1*, Chaturvedi S1, Jha DK2 and Chaturvedi M3
1Department of Pathology, Institute of Human Behavior and Allied Sciences, Delhi, India
2Department of Neurosurgery, Institute of Human Behavior and Allied Sciences, Delhi, India
3Department of Neuroradiology, Institute of Human Behavior and Allied Sciences, Delhi, India
- *Corresponding Author:
- Ishita Pant
Department of Pathology
Institute of Human Behavior and Allied Sciences
Delhi, 110 095, India
Tel: +91 9868396863
E-mail: ishitapant@gmail.com
Received date: March 14, 2019; Accepted date: April 17, 2019; Published date: April 22, 2019
Citation: Pant I, Chaturvedi S, Jha DK, Chaturvedi M (2019) Glioblastoma in a Male with Down’s Syndrome: A Case Report. J Neoplasm. Vol.4 No.1:1 doi:10.21767/2576-3903.100135
Abstract
Tumors of the Central Nervous System (CNS) are rarely reported in patients of Down’s syndrome. The authors report a case of glioblastoma in a 27-year-old male who had Down’s syndrome. An intensive literature search by the authors showed 36 cases of CNS tumors associated with Down’s syndrome, amongst this only 1 case was reported as glioblastoma. To the best of our knowledge, this is the second case report of glioblastoma associated with Down’s syndrome.
Keywords
Glioblastoma; Down’s syndrome; Central nervous system tumors
Abbreviations
CNS: Central Nervous System; IQ: Intelligence Quotient; MRI: Magnetic Resonance Imaging
Introduction
Down’s syndrome is the commonest genetic chromosomal disorder and is found in one out of every 700 new-borns. As a well-known fact, individuals with Down’s syndrome are predisposed to certain neoplasms, amongst which leukemia and other hematological malignancies are the commonest followed by testicular tumors [1]. However, other solid tumors are generally uncommon [2,3]. Case reports of Down’s syndrome with associated CNS tumors are sparse in the literature [4]. The authors report a case of glioblastoma in a 27-year old male a known case of Down’s syndrome. To the best of our knowledge, this is the second case report of glioblastoma associated with Down’s syndrome. In this case report, we discuss the association of glioblastoma and other CNS tumors with Down’s syndrome [5].
Case Report
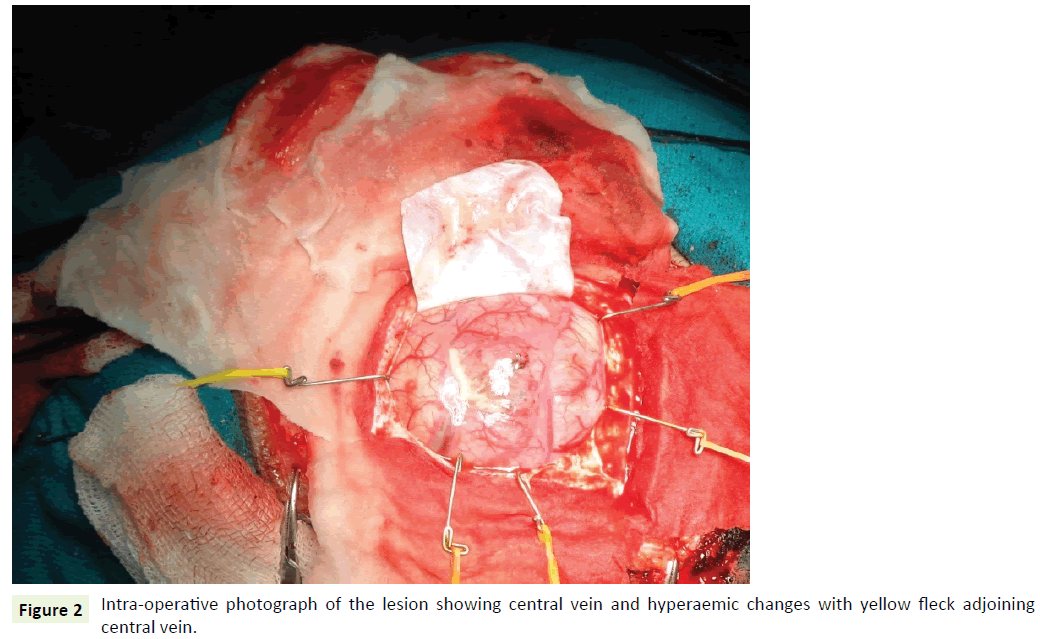
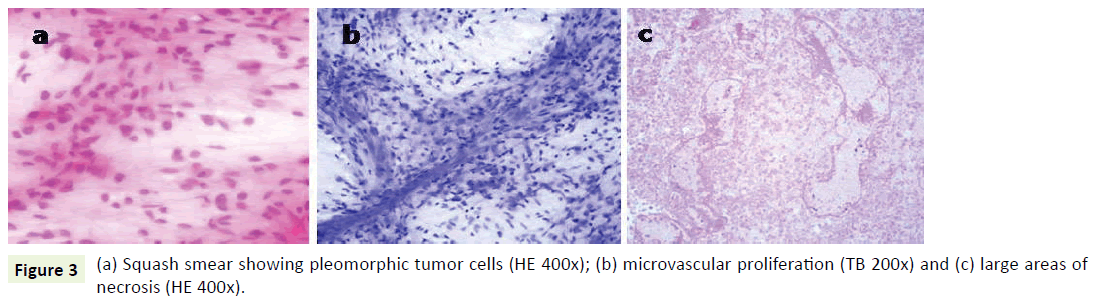
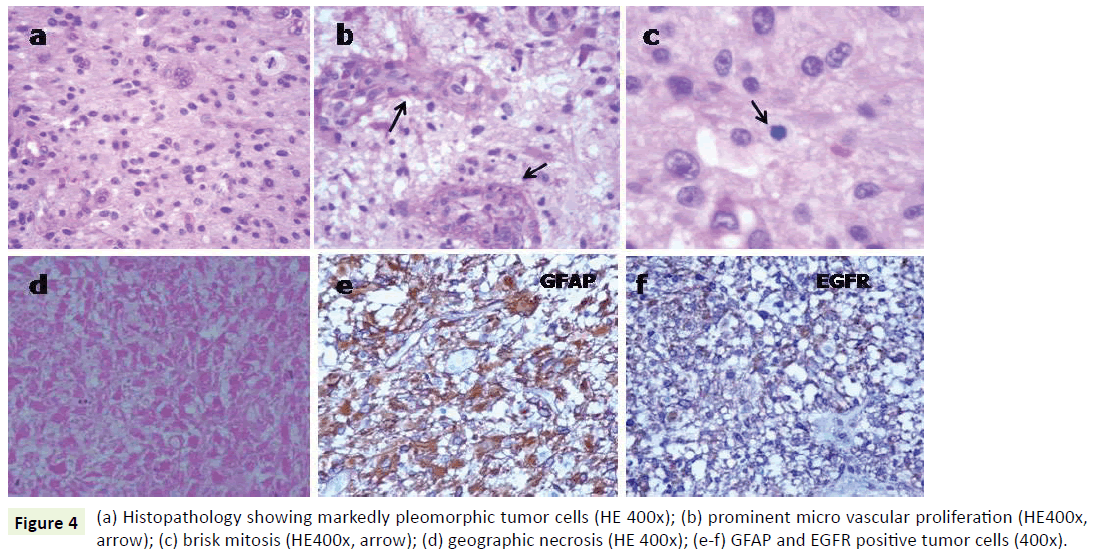
A 27-year-old male a known case of Down’s syndrome (21 Trisomy, 47, XY, +G), presented with a short history of headache and vomiting for the last 15-20 days with associated progressive weakness of left upper and lower limbs. On examination, he had minimal left hemiparesis with a motor power of grade 4 in both upper and lower limbs on the left side. His serial Intelligence Quotient (IQ) assessments were 50 to 55 and reevaluation revealed it in the same range. Magnetic Resonance Imaging (MRI) revealed a well-defined, large, rounded mass in the right parietotemporal lobe (Figure 1a). The mass showed thick irregular enhancing margins, central necrosis and moderate perilesional edema effacing the overlying sulci and right Sylvian fissure. There was a midline shift to the left showing compression of the right lateral ventricle (Figure 1b and 1c). Based on these, a provisional radiological diagnosis of glioma was considered. The lesion was approached by a right parietal craniotomy, exposing a hyperemic lesion on the post-central gyrus with yellowish flecks adjoining central vein (Figure 2). Intratumoral decompression was done which revealed the solid and cystic components of the mass along with necrotic areas. Gross total tumor removal was done. The per-operative specimen sent to the histopathology laboratory was subjected to squash smear preparation and frozen sections. Squash smears and frozen sections revealed pleomorphic fibrillary astrocytes (Figure 3a), microvascular proliferation (Figure 3b) and prominent necrotic foci (Figure 3c). Based on the above findings a perioperative diagnosis of a highgrade glioma was suggested. The latter specimen received in formalin for paraffin sections showed a cellular astrocytic tumor comprised of markedly pleomorphic astrocytes (Figure 4a) along with large areas of geographic necrosis (Figure 4b) and marked microvascular proliferation (Figure 4c). Brisk mitosis was frequent (Figure 4d). Tumor cells showed immunopositivity for GFAP and EGFR (Figure 4e and 4f). This case was reported as glioblastoma (WHO grade IV).
The postoperative course was uneventful, the postoperative scan revealed no residual tumor. The patient was referred to as radiotherapy. Radiotherapy was started, however, despite of multimodality approach the patient’s general condition deteriorated day by day and he died after one month.
Discussion
The authors report a case of glioblastoma in a known case of Down’s syndrome. To the best of our knowledge, this is the second case report of glioblastoma associated with Down’s syndrome. Overall CNS tumors in individuals with Down’s syndrome are extremely rare. A review of neoplasms in Down’s syndrome conducted by Stage et al. concluded that leukemia’s, lymphomas and germ cell tumors were the commonly encountered neoplasms in patients with Down’s syndrome. This review further stated that Down’s syndrome has a particular tumor profile with germ cells and mesenchymal tissue appearing more prone to tumor development whereas central and peripheral nervous system, renal tissue and epithelial tissues appeared resistant to neoplastic transformation [4]. Stage et al. in their next series reported 2 spinal tumors and 36 intracranial tumors in patients with Down’s syndrome. Amongst the 36 intracranial tumors only 1 case of glioblastoma was reported. A recent Japanese study published, reported 114 tumors in a total 1514 patients of Down’s syndrome, out of which 87 cases were reported as hematological malignancies; 17 cases included various solid tumors while 10 cases were reported as benign tumors [5]. Only 3 cases of CNS tumors were reported in this series.
In the recent past, genetic studies have explained this typical tumor profile in Down’s syndrome. Mutations in GATA1 gene (which encodes the essential transcription factor required for the development of megakaryocytes and other hematopoietic cells) have been identified resulting in myeloproliferative disorders and acute megakaryoblastic leukemia [3]. Similarly, JAK2 mutations have been identified in patients of Down’s syndrome with associated acute lymphoblastic leukemia [6]. Further, it has been suggested that dysregulated expressions of antiangiogenic genes (COL18A, Adam TS1, DCR1) on chromosome 21 leading to elevated endogenous angiogenesis inhibitors may be attributed to the rarity of solid tumors in Down’s syndrome [7]. Though the molecular regulation of angiogenesis is not yet fully understood, however, further studies may give us insights toward novel therapies [8].
Conclusion
Tumor profile studies in Down’s syndrome patients’ shows a propensity for lymphoma, leukemia, and germ cell tumors. The central and peripheral nervous system, renal tissue and epithelial tissues are relatively resistant to neoplastic transformation in Down’s syndrome patients. This variable propensity for different types of tumors has been explained genetically. This case has been reported for its rarity and, to emphasize the need to record and analyze the pathogenetic mechanisms involved in such exceptions.
References
- Parker SE, Mai CT, Canfield MA, Rickard R, Yang Y et al. (2010) Updated national birth prevalence estimates for selected birth defects in the United States 2004-2006. Birth Defects Res A Clin Mol Teratol 88: 1008-1016.
- Powers LW, Register MK (1991) Down syndrome and acute leukaemia: epidemiological and genetic relationships. Lab Med 22: 630-636.
- Ehara H, OhnoK, Ito H (2011) Benign and malignant tumors in Down syndrome: analysis of the 1514 autopsied cases in Japan. Pediatrint 53: 72-77.
- Satge D, Sommelet D, Geneix A, Nishi M, Malet P et al. (1998) A tumor profile in Down syndrome. Am J Med Genet 78:207-216.
- Satge D, Monteil P, Sasco AJ, Vital A, Ohgaki , et al. (2001) Aspects of intracranial and spinal tumors in patients with Down syndrome and report of a rapidly progressing grade 2 astrocytoma. Cancer 91: 1458-1466.
- Rainis L, Bercovich D, Strehl S, Teigler SA, Stark B et al. (2003) Mutations in exon 2 of GATA1 are early events in megakaryocytic malignancies associated with Trisomy 21. Blood 102: 981-986.
- Bercovich D, Ganmore I, Scott LM, Wainreb G, Birger Y et al. (2008) Mutations of JAK2 in acute lymphoblastic leukaemia’s associated with Down’s syndrome. Lancet 372: 1484-1492.
- Ryeom S, Folkman J (2009) Role of endogenous angiogenesis inhibitors in Down syndrome. J Craniofac Surg 20: 595-596.

Open Access Journals
- Aquaculture & Veterinary Science
- Chemistry & Chemical Sciences
- Clinical Sciences
- Engineering
- General Science
- Genetics & Molecular Biology
- Health Care & Nursing
- Immunology & Microbiology
- Materials Science
- Mathematics & Physics
- Medical Sciences
- Neurology & Psychiatry
- Oncology & Cancer Science
- Pharmaceutical Sciences