Multiple Endocrine Neoplasia Type 2A Patient with Metastatic Medullary Thyroid Carcinoma and Unilateral Multicentric Pheochromocytoma: A Case Report and Literature Review
Harrison D Martin, Preeti Behl, Abida Kadi, Rachna Jetly-Shridhar, Emad Kandil and Nadja K Falk
Published Date: 2018-06-10DOI10.21767/2576-3903.100032
Harrison D Martin1, Preeti Behl1, Abida Kadi1, Rachna Jetly-Shridhar2, Emad Kandil3 and Nadja K Falk1*
1Department of Pathology and Laboratory Medicine, Tulane School of Medicine, New Orleans, USA
2Department of Pathology, Louisiana State University School of Medicine, New Orleans, USA
3Division of Endocrine and Oncological Surgery, Tulane School of Medicine, New Orleans, USA
- *Corresponding Author:
- Falk NK
Department of Pathology and Laboratory Medicine, Tulane School of Medicine, 1430 Tulane Ave., New Orleans, LA 70112 USA.
Tel: 504-988-2972
E-mail: Nfalk1@tulane.edu
Received: May 18, 2018; Accepted: June 06, 2018; Published: June 10, 2018
Citation: Martin HD, Behl P, Kadi A, Shridhar RJ, Kandil E, et al. (2018) Multiple Endocrine Neoplasia Type 2A Patient with Metastatic Medullary Thyroid Carcinoma and Unilateral Multicentric Pheochromocytoma: A Case Report and Literature Review. J Neoplasm. Vol.3 No.2:7 doi: 10.21767/2576-3903.100032
Abstract
Introduction: Multiple endocrine neoplasia 2A (MEN2A) is an autosomal dominant syndrome associated with germline mutations in the RET proto-oncogene that may lead to medullary thyroid carcinomas (MTC), pheochromocytomas, and parathyroid adenomas. Patients generally present between the ages of 20 and 30 years. MEN2 patients are stratified for risk based upon the RET mutation, and a genotype-phenotype correlation exists.
Case Report: We describe a 36-year-old male recently diagnosed with MEN2A syndrome and RET oncogene mutation [c.1900T>C;p.Cys634Arg], which is a high risk category. He had elevated calcitonin, calcium, parathyroid hormone, and alkaline phosphatase levels with decreased ionized calcium. 24-hour urine testing showed elevated metanephrines and catecholamines. Imaging studies showed a right adrenal mass and multicentric thyroid nodules with a mass adherent to the right inferior posterior thyroid lobe. Right adrenalectomy histologic evaluation revealed multicentric pheochromocytomas in the right adrenal gland. Total thyroidectomy with bilateral central and lateral neck dissections confirmed multicentric MTC metastatic to one right lateral neck lymph node. Although only one parathyroid gland was removed, the findings were suggestive of parathyroid adenoma.
Discussion and Conclusion: This case is remarkable for the relatively advanced patient age for presentation with this syndrome given the high-risk category as well as multifocality of the associated neoplasms. The general pathologist should be aware of the MEN2 risk stratification categories based on molecular profiling and the implications on patient care.
Keywords
Multiple endocrine neoplasia; Multiple endocrine neoplasia type 2A; RET; Medullary thyroid carcinoma; Pheochromocytoma
Abbreviations
FMTC - familial medullary thyroid carcinoma; HD - Hirschsprung disease; MEN - Multiple endocrine neoplasia syndrome; MTC - medullary thyroid carcinoma.
Introduction
Multiple endocrine neoplasia syndromes (MEN) exhibit neoplasms in various organs due to different genetic mutations. Jacob Erdheim described the first case of MEN in 1903 [1]. In 1968 Steiner et al. described MEN type 1 (MEN1) and MEN type 2 (MEN 2) [2]. In 1973 MEN types 2A (MEN2A) and MEN type 2B (MEN2B) were described [3,4].
MEN1 (Wermer syndrome) is associated with parathyroid adenomas, pancreatoenteric neuroendocrine tumors, anterior pituitary tumors, and adrenal cortical tumors. MEN1 patients generally present at a mean age of 16 years, most commonly with hyperparathyroidism. MEN2 has an estimated prevalence of 2.5 per 100,000 in the general population [5]. MEN2 includes MEN2A (Sipple’s syndrome), of which familial medullary thyroid carcinoma (FMTC) is a category, and MEN2B. MEN2A is the most common MEN2 syndrome and comprises 55% of cases. MEN2A is associated with medullary thyroid carcinomas, pheochromocytomas, and parathyroid adenomas. MEN2 patients have a 70% to 100% risk of MTC by age 70-years [6]. MEN2 patients have pheochromocytoma as the first clinical presentation in only 10% to 30% of patients [7]. FMTC is the mildest variant of MEN2 and is seen in 10% to 20% of cases. FMTC should be considered when four family members have MTC only. MEN2B, which is more aggressive and associated with an earlier presentation, comprises 5-10% of MEN2 cases and is associated with MTC, pheochromocytomas, mucosal ganglioneuromas, and marfanoid body habitus. MEN4 patients present similarly to MEN1 patients but appear to have a different genetic mechanism. These patients are often diagnosed later in life (30 to 74 years) and usually have hyperthyroidism and may have parathyroid adenomas. A variety of endocrine and nonendocrine neoplasms may be seen in Table 1 [5,8].
| Characteristics | MEN1 | MEN2A | MEN2B | MEN4 |
|---|---|---|---|---|
| Types of endocrine neoplasms | Parathyroid adenomas (90%) | MTC (90%) | MTC (100%) | Parathyroid adenomas (majority) |
| Pancreatoenteric NETs (30-70%) | Pheochromocytomas (50%) | Pheochromocytomas (50%) | ||
| Anterior pituitary tumors (30-40%) | Paragangliomas (50%) | Small cell neuroendocrine cervical carcinoma | ||
| Adrenal cortical tumors (40%) | Parathyroid adenomas (20-30%) | Adrenal masses | ||
| FMTC Variant: MTC 95% | Bronchial carcinoid | |||
| Papillary thyroid carcinoma | ||||
| Gastric carcinoid | ||||
| Pancreatoenteric masses | ||||
| Non-endocrine findings | Lipomas | Cutaneous lichenoid amyloidosis | Mucosal ganglioneuromas | Angiomyolipoma |
| angiofibromas | Schwannoma | |||
| Hirschsprung disease | Marfanoid body habitus | Meningioma | ||
| collagenomas | Liver hemangioma | |||
| ependymomas | Prostate cancer | |||
| Breast cancer | ||||
| Lipoma | ||||
| Uterine fibroids |
Note: MEN- Multiple endocrine neoplasia, NET-Neuroendocrine tumor, MTC-Medullary thyroid carcinoma, FMTC-Familial medullary thyroid carcinoma
Table 1: Clinical characteristics of MEN syndrome types.
MEN2A generally first manifests with MTC between the ages of 20-30 years of age. MTC, which accounts for 1-2% of thyroid cancers in the United States, has a 90% penetrance in MEN2A patients. MTC originates from thyroid C-cells, which secrete the 32-amino-acid glycoprotein calcitonin and are more predominant in the upper poles of the right and left lobes. Calcitonin is a serologic tumor marker for MTC. The nests of tumor cells, which may be spindled, have neuroendocrine features (“salt and pepper” chromatin) and a fibrous background which may contain amyloid. MTC metastases are most commonly found in the cervical lymph nodes, however metastases have also been identified in lung, liver and bone. Pheochromocytomas and paragangliomas have a 50% penetrance in MEN2A patients and can cause hypertension, headaches, sweating and palpitations due to catecholamine production. Pheochromocytomas develop in the medulla of the adrenal gland and are commonly bilateral in MEN2. Paragangliomas develop in the paraganglia. Both show nests of cells (“Zellballen”) surrounded by spindled sustentacular cells. MEN2 patients with unilateral pheochromocytoma generally develop contralateral pheochromocytoma within 10 years [9]. Non-endocrine findings in MEN2A may include Hirschsprung disease (HD) and cutaneous lichen amyloidosis [10]. HD presents in the first few days of life and is diagnosed by a lack of ganglion cells in the distal intestinal wall. HD is associated with RET codon 10 mutations and may exist without other MEN2A symptoms. Cutaneous lichen amyloidosis presents with itching, papules, and hyperpigmentation in the upper mid back; histologically amyloid is present [5,8,9,11].
We present a MEN2A patient of advanced age (for this syndrome). The general pathologist should be aware of the manifold presentations of MEN syndromes. The genetic mutation results are of particular interest in regard to genotype-phenotype correlation and risk group stratification.
Case Presentation
A 36-year-old male presented normotensive with occasional sharp headaches and slight weight loss. The patient’s sister was diagnosed five years prior with MEN2A syndrome. Laboratory testing showed elevated calcitonin (123.0 pg/mL), calcium (11.2 MG/DL), parathyroid hormone level (2035 PG/ML), and alkaline phosphatase (1221 U/L). 24-hour urine testing showed elevated metanephrines (291 ug/d) and catecholamines (630 ug/24 hr). Ionized calcium was decreased (3.2 mg/dL).
Neck ultrasound revealed bilateral thyroid nodules (right – 1.2 cm and left – 1.4 cm in greatest dimension) and a 3.5 cm mass at the posterior inferior aspect of the right thyroid gland. A computed tomography of the abdomen revealed a hyperdense 1.2 cm nodule of the right adrenal gland and ill-defined nodularity of the left adrenal gland. Metaiodobenzylguanidine scan indicated increased radiotracer uptake in the adrenal glands bilaterally and left anterior neck.
Pathologic Findings
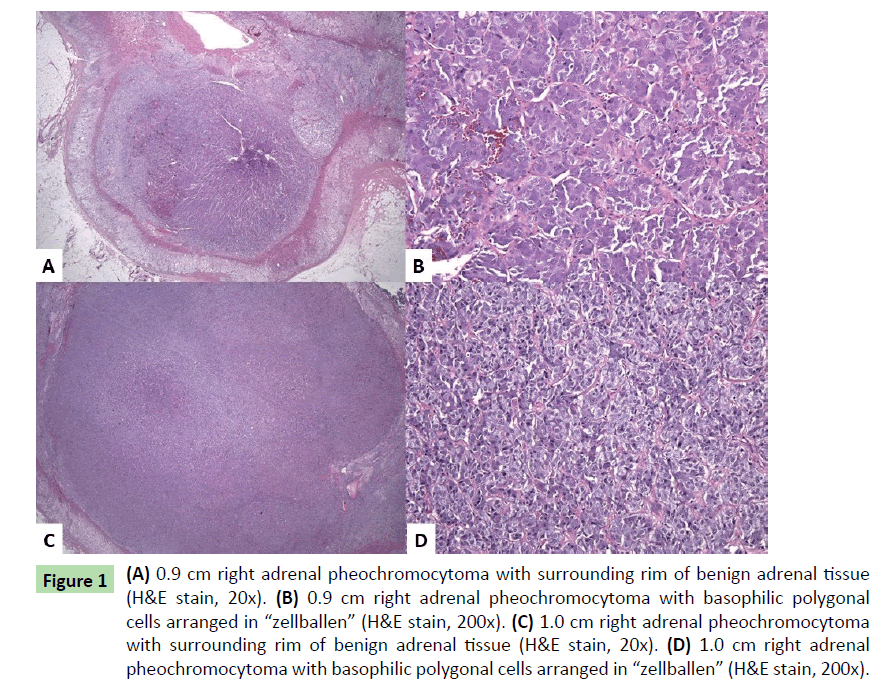
Results of familial mutation targeted testing (ARUP Laboratories, Salt Lake City, Utah) performed on the patient’s serum one year prior to presentation revealed RET oncogene mutation [c.1900T>C;p.Cys634Arg], confirming MEN2A. Fifteen months after this diagnosis, the patient underwent right adrenalectomy. An enlarged tan-yellow adrenal gland (12.3 g) measuring 5.6 × 2.7 × 1 cm overall revealed two separates yellow – brown nodules, 0.9 and 1.0 cm in greatest dimension. Neither lesion appeared grossly to invade through the cortex or into the periadrenal fat. Histologic examination (H&E slides) of both nodules showed similar features: polygonal-shaped cells with abundant basophilic cytoplasm and round nuclei with nucleoli and clearing. The cells were arranged in nests (“zellballen”) (Figures 1a-1d). Immunohistochemical stains showed the tumor cells to be positive for synaptophysin and chromogranin. S-100 highlighted sustentacular cells surrounding the “zellballen”. The Ki-67 proliferation index was less than 1%. No significant cytologic atypia, capsular or vascular invasion, significant mitotic activity, or necrosis was noted. The findings were consistent with multifocal pheochromocytomas of the right adrenal gland.
Figure 1: (A) 0.9 cm right adrenal pheochromocytoma with surrounding rim of benign adrenal tissue (H&E stain, 20x). (B) 0.9 cm right adrenal pheochromocytoma with basophilic polygonal cells arranged in “zellballen” (H&E stain, 200x). (C) 1.0 cm right adrenal pheochromocytoma with surrounding rim of benign adrenal tissue (H&E stain, 20x). (D) 1.0 cm right adrenal pheochromocytoma with basophilic polygonal cells arranged in “zellballen” (H&E stain, 200x).
One month following his right adrenalectomy, the patient underwent total thyroidectomy with bilateral central and lateral neck dissections. The thyroid lobes were submitted separately and were enlarged. The right thyroid lobe weighed 25 g and measured 5.4 × 3.4 × 1.6 cm. The left thyroid lobe weighed 9 g and measured 4.9 × 2.5 × 1.3 cm. Grossly, bilateral gray-yellow firm nodules were seen (right-1.4 cm, upper pole; left-0.9 cm, upper pole). A 3.6 × 2.9 × 1.2 cm tan nodule was attached to the inferior posterior right thyroid gland. Six lymph nodes were identified in the right central neck compartment; five, in the left central neck compartment; thirty-four, in the right lateral neck; and twenty, in the left lateral neck.
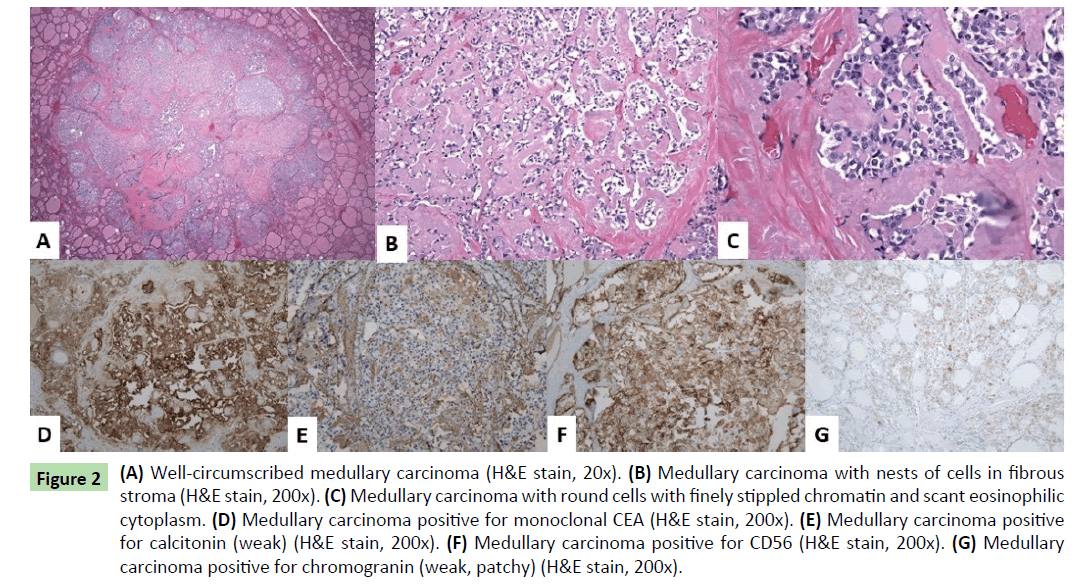
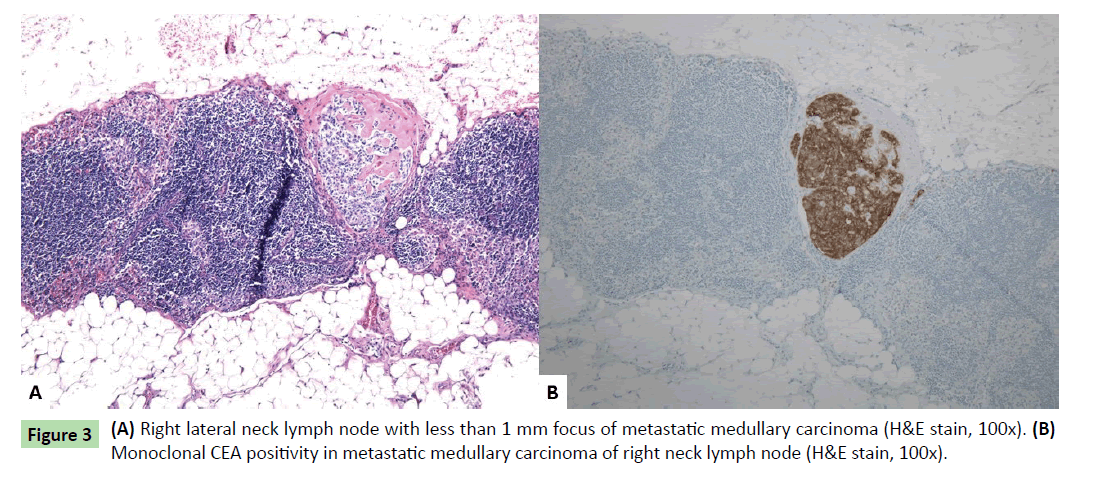
Histologically, one additional right thyroid nodule was identified, measuring 0.1 cm. The three thyroid masses (two in right thyroid lobe and one in left thyroid lobe) had similar histologic features. Well-circumscribed nodules were seen with nests of round cells among fibrous stroma (Figure 2a). The nuclei had finely stippled (neuroendocrine-type) chromatin and scant eosinophilic cytoplasm (Figures 2b and 2c). Immunohistochemical stains showed the tumor cells to be positive for monoclonal CEA, calcitonin (weak), CD56, and chromogranin (weak, patchy) (Figures 2d-2g). Rare tumor cells were positive for TTF-1. The findings were consistent with multifocal medullary carcinoma. The surgical margins were free of tumor. One lymph node in the right lateral neck contained a less than 1 mm focus of metastatic medullary carcinoma positive for monoclonal CEA immunohistochemically (Figures 3a and 3b). The remainder of the lymph nodes contained no metastatic carcinoma.
Figure 2 (A) Well-circumscribed medullary carcinoma (H&E stain, 20x). (B) Medullary carcinoma with nests of cells in fibrous stroma (H&E stain, 200x). (C) Medullary carcinoma with round cells with finely stippled chromatin and scant eosinophilic cytoplasm. (D) Medullary carcinoma positive for monoclonal CEA (H&E stain, 200x). (E) Medullary carcinoma positive for calcitonin (weak) (H&E stain, 200x). (F) Medullary carcinoma positive for CD56 (H&E stain, 200x). (G) Medullary carcinoma positive for chromogranin (weak, patchy) (H&E stain, 200x).
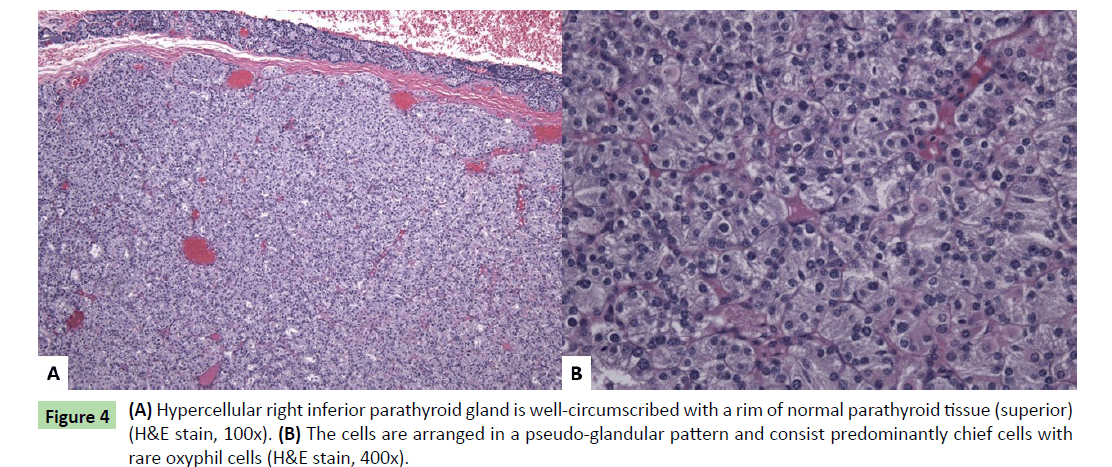
The mass attached to the inferior right posterior thyroid gland had a smooth tan-brown surface and a homogeneous tan-yellow cut surface. Histologic sections revealed a well-circumscribed nodule consisting primarily of chief cells with a rim of normal compressed parathyroid tissue (Figure 4a). The cells were arranged in a pseudo-glandular pattern, and rare oxyphil cells were seen (Figure 4b). Essentially no adipose tissue was seen, and no mitotic activity or atypia was observed. The findings showed hypercellular parathyroid tissue suggestive of adenoma. The other three parathyroid glands were not sampled; however, they were not enlarged radiologically nor was enlargement noted by the surgeon.
The postoperative course was unremarkable, and the patient now takes calcitriol, levothyroxine, and calcium carbonate. He continues to be monitored by his endocrine subspecialist for management of care.
Discussion
Although sporadic cases can occur, MEN syndrome inheritance is usually autosomal dominant, and offspring have a 50% risk of inheriting the disease. MEN1 is associated with MEN1 gene mutations (germline and somatic) which include frameshift and nonsense mutations, in-frame deletions, and missense alterations which have a tumor suppressor effect. Originally described in 1985, all MEN2 types are associated with germline RET proto-oncogene mutations, later mapped to chromosome 10q11.2 [12,13]. MEN4 is associated with CDKN1B mutations, commonly p27 (Table 2) [8,9,14,15].
| Mutation | MEN1 | MEN2A | MEN2B | MEN4 |
|---|---|---|---|---|
| Genetic Mutations | MEN1 gene menin | RET gene (95%) germline mutations in codon 634 exon 11 or codons 609, 611, 618 or 620 exon 10 | RET gene (95%) germline mutation in codon 918 | CDKN1B |
| chromosome 11q13 |
Note: MEN-Multiple endocrine neoplasia.
Table 2: Most common genetic mutations associated with MEN syndrome types.
Neuroendocrine cells, which include thyroid C-, parathyroid, and pancreatic medullary cells, express RET. Missense mutations in the RET proto-oncogene resulting in a gain of function cause MEN2, and over 100 different mutations have been identified. The RET gene, which has 21 exons, encodes receptor tyrosine kinase causing changes in growth and differentiation during development. The RET protein has a ligand-binding domain extracellular segment, a calcium-dependent cadherin-like domain, and a cysteine-rich domain nearest the cell membrane. The single transmembrane domain connects to the intracellular component, which has two tyrosine kinase subdomains. The glial cell-derived neurotrophic factor family of ligands use RET as their signaling receptor [16]. Common mutations are those of cysteine residues in the extracellular domain, which cause dimerization of the receptor in the absence of a ligand. This leads to autophosphorylation of RET, activation of downstream signaling pathways, and varying presentations of MEN syndromes [13,16,17]. With MEN 2A, 95% of patients have germline mutations in codon 634 of exon 11 and in codons 609, 611, 618 or codon 620 exon 10. Somatic RET mutations, particularly M918T are seen in 40-50% of sporadic MTC. Pheochromocytoma penetrance is 50% with codon 634 mutation carriers, approximately 20% with exon 10 mutation carriers, and less than 5% in exon 13-15 mutation carriers [18,19]. Primary hyperparathyroidism is seen in 30-20% of MEN2A patients with RET 634 mutations and 2-5% with exon 10 mutations [19,20]. FMTC syndrome is associated with lowrisk non-cysteine mutations in exons 13-15. 95% of MEN2B cases are due to a germline RET mutation in codon 918, the majority of which are de novo mutations of paternal origin B [21]. More indolent MEN2B is associated with RET mutation at codon 883 [22,23].
MEN2 patients are stratified for risk based upon the RET mutation, and genotype-phenotype correlation exists [24]. The highest risk mutation is RET M819T, which is associated with MEN2B and causes aggressive MTC at a young age. Metastatic MTC has been seen as early as 3 months of age with this mutation. The highrisk group includes RET codon 634 mutations of exon 11, which are associated with early age onset of MTC, and MTC has been detected at age 10 months [25]. In patients younger than 10 years, the MTC is rarely metastatic [26]. Hyperparathyroidism has a moderate penetrance with RET codon 634 mutations [9]. The moderate risk group is associated with the following mutations: exon 10 RET 609, 611, 618, 620 and exons 13-15. These lower risk mutations typically present with MTC later in life and with a less aggressive course [27]. Exon 10 mutations have a median age of MTC between 20 and 40 years of age, and metastases generally occur over 30 years of age [19]. Pheochromocytomas have a lower rate of penetrance with exon 10 RET codon mutations and are usually benign, multicentric, bilateral and confined to the adrenal gland. Hyperparathyroidism has a 2-12% penetrance with RET codon 609, 611, 618, and 620 mutations [9,23,28-30].
MEN2 surveillance and treatment emphasizes early diagnosis and treatment of MTC [6]. Prophylactic early thyroidectomies based on genetic mutational analysis in MEN2 has improved survival [23]. The highest risk group MEN2B pediatric patients with the most common M918T mutation should undergo prophylactic thyroidectomy before age 1 with level VI lymph node dissection if possible. Physical exam, neck ultrasound and serum calcitonin and carcinoembryonic antigen levels (including doubling times) are checked every 6 months and then annually. At age 11, pheochromocytoma screening should be initiated. MEN2A patients should have pheochromocytomas removed prior to thyroidectomy [5]. The MEN2A pediatric high-risk group should have prophylactic thyroidectomy at or before age five based on calcitonin level. The semi-annual and annual checks as well as pheochromocytoma screening are the same as for the highest risk group. Pediatric MEN2A moderate risk patients should have prophylactic thyroidectomy when calcitonin becomes elevated or at the parents’ discretion. Pheochromocytoma screening is initiated at age sixteen. Adults with RET germline mutations should have annual calcitonin level screening. If this level becomes elevated, prophylactic thyroidectomy with lymph node dissection should be performed as well as pheochromocytoma screening [9]. After total thyroidectomy, the patients are treated with thyroxine replacement to normalize the thyroid-stimulating hormone level [31]. Tyrosine kinase inhibitors may be considered for unresectable, locally advanced and progressive metastatic MTC [32]. MEN1 pediatric patients should have screening for parathyroid, pituitary, and pancreatic neoplasms beginning less than ten years of age [33]. The most frequent cause of MEN1- related death is pancreatoenteric NET complications [8].
All first-degree relatives of MEN patients should have genetic testing, which detects nearly 100% of carriers of mutations [5,23]. RET analysis most commonly includes DNA sequencing of exons 5,8,10,11, and 13-16 [23]. Patients diagnosed with MEN syndromes should have aggressive follow-up including imaging and additional genetic biomarker testing [8].
Conclusion
We described a molecular category high risk patient who presented at advanced age with newly diagnosed MEN2A syndrome. Serologic and urine testing, radiologic scanning, physical examination and history helped detect evidence of thyroid, parathyroid, and adrenal neoplasms. Histologically, bilateral, multifocal, metastatic MTC and unilateral multicentric pheochromocytoma were identified. The patient will have close follow-up. RET mutation analysis has become more common particularly with MTC. The general pathologist should be aware that with the increased molecular understanding of MEN2 cancer syndromes comes a concomitant need to understand the differing genetic profiles of the numerous RET mutations and the implications on patient care.
References
- Carney JA (2005) Familial multiple endocrine neoplasia: The first 100 years. Am J Surg Pathol 29(2): 254-274.
- Steiner AL, Goodman AD, Powers SR (1968) Study of kindred with pheochromocytoma, medullary thyroid carcinoma, hyperparathyroidism and Cushing’s disease: Multiple endocrine neoplasia, type 2 Medicine (Baltimore). 47(5): 371-409.
- Sizemore GW, Health H, Carney JA (1980) Multiple endocrine neoplasia type 2. Clin Endocrin Metab 9(2): 299-315.
- Almeida MQ, Stratakis CA (2010) Solid tumors associated with multiple endocrine neoplasias Cancer Genet Cytogenet 203(1): 30-36.
- Raue F, Frank-Raue K (2010) Update multiple endocrine neoplasia type 2. Familial Cancer 9(3): 449-457.
- Krampitz GW, Norton JA (2014) RET gene mutations (genotype and phenotype) of multiple endocrine neoplasia type 2 and familial medullary thyroid carcinoma. Cancer 120(13): 1920-1931.
- Lefebvre M, Foulkes WD (2014) Pheochromocytoma and paraganglioma syndromes: Genetics and managements update. Curr Oncol 21(1): e8-e17.
- Pacheco MC (2016) Multiple endocrine neoplasia: A genetically diverse group of familial tumor syndromes. J Pediatr Genet 5 (2): 89-97.
- Wells SA Jr, Asa SL, Dralle H, Elisei R, Evans DB, et al. (2015) Revised american thyroid association guidelines for the management of medullary thyroid carcinoma. Thyroid 25(6): 567-610.
- Cohen MS, Phay JE, Albinson C, DeBenedetti MK, Skinner MA, et al. (2002) Gastrointestinal manifestations of multiple endocrine neoplasia type 2. Ann Surg 235(5): 648-654.
- Verga U, Fugazzola L, Cambiaghi S, Cambiaghi S, Pritelli C, et al. (2003) Frequent association between MEN 2A and cutaneous lichen amyloidosis. Clin Endocrinol (Oxf) 59(2): 156-161.
- Simpson NE, Kidd KK, Goodfellow PJ, McDermid H, Myers S, et al. (1987) Assignment of multiple endocrine neoplasia type 2A to chromosome 10 by linkage. Nature 328(6130): 528-530.
- Traugott AL, Moley JF (2010) The RET protooncogene. Cancer Treat Res 153: 303-319.
- Molatore S, Mariononi I, Lee M, Pulz E, Ambrosio MR, et al. (2010) A novel germline CDKN1B mutation causing multiple endocrine tumors: Clinical, genetic and functional characterization. Hum Mutat 31(11): E1825-E1835.
- Occhi G, Regazzo D, Trivellin G, Boaretto F, Ciato D, et al. (2013) A novel mutation in the upstream open reading frame of the CDKN1B gene causes a MEN4 phenotype. PloS Genet 9(3): e1003350.
- Plaza-Menacho I, Mologni L, McDonald NQ (2014) Mechanisms of RET signaling in cancer: Current and future implications for targeted therapy. Cell Signal 26(8): 1743-1752.
- Wohllk N, Schweizer H, Erlic Z, Schmid KW, Walz MK, et al. (2010) Multiple endocrine neoplasia type 2. Best Pract Res Clin Endocrinol Metab 24(3): 371-387.
- Quayle FJ, Fialkowski EA, Benveniste R, Moley JF (2007) Pheochromocytoma penetrance varies by RET mutation in MEN 2A. Surgery 142(56): 800-805.
- Frank-Raue K, Rybicki LA, Erlic Z, Schweizer H, Winter A, et al. (2011) Risk profiles and penetrance estimations in multiple endocrine neoplasia type 2A caused by germline RET mutations located in exon 10. Hum Mutat 32(1): 51-58.
- Schuffenecker I, Virally-Monod M, Brohet R, Goldgar D, Conte-Debolx B, et al. (1998) Risk and penetrance of primary hyperparathyroidism in multiple endocrine neoplasia type 2A families with mutations at codon 634 of the RET proto-oncogene. Groupe E’etude des Tumeurs a Calcitonine. J Clin Endocrinol Metab 83(2): 487-491.
- Braukhoff M, Machens A, Hess S, Lorenz K, Gimm O, et al. (2008) Premonitory symptoms preceding metastatic medullary thyroid cancer in MEN 2B: an exploratory analysis. Surgery 144(6): 1044-1050.
- Jasim S, Ying AK, Waguesepack SG, Rich TA, Grubbs EG, et al. (2011) Multiple endocrine neoplasia type 2B with a RET proto-oncogene A883F mutation displays a more indolent form of medullary thyroid carcinoma compared with a RET M918T mutation. Thyroid 21(2): 189-192.
- Frank-Raue K, Raue F (2015) Hereditary medullary thyroid cancer genotype-phenotype correlation. Recent Results Cancer Res 204: 139-156.
- Raue F, Frank-Raue K (2012) Genotype-phenotype correlation in multiple endocrine neoplasia type 2. Clinics (Sao Paulo) 67 Suppl 1: 69-75.
- Zenaty D, Aigrain Y, Peuchmaur M, Philippe-Chomette P, Baumann C, et al. (2009) Medullary thyroid carcinoma identified within the first year of life in children with hereditary multiple endocrine neoplasia type 2A (codon 634) and 2B. Eur J Endocrinol Eur Fed Endocr Soc 160(5): 807-813.
- Machens A, Niccoli-Sire P, Hoegel J, Frank-Raue K, van Vroonhoven TJ, et al. (2003) Early malignant progression of hereditary medullary thyroid cancer. N Engl J Med 349(16): 1517-1525.
- Rich TA, Feng L, Busaidy N, Cote GJ, Gagel RF, et al. (2014) Prevalence by age and predictors of medullary thyroid cancer in patients with lower risk germline RET proto-oncogene mutations. Thyroid 24(7): 1096-1106.
- Mulligan LM, Marsh DJ, Robinson BG, Schuffenecker I, Zedenius J, et al. (1995) Genotype-phenotype correlation in multiple endocrine neoplasia type 2: Report of the International RET Mutation Consortium. J Intern Med 238(4): 343-346.
- Brandi ML, Gagel RF, Angeli A, Bilezikian JP, Beck-Peccoz P, et al. (2001) Consensus guidelines for diagnosis and therapy of MEN type 1 and type 2. J Clin Endocrinol Metab 86(12): 5658-5671.
- Kloos RT, Eng C, Evans DB, Francis GL, Gagel RF, et al. (2009) Medullary thyroid cancer: Management guidelines of the american thyroid association. Thyroid 19(6).
- Pacini F, Castagna MG, Cipri C, Schlumberger M (2010) Medullary thyroid carcinoma. Clin Oncol (R Coll Radiol) 22(6): 475-485.
- Pappa T, Alevizaki M (2016) Management of hereditary medullary thyroid carcinoma. Endocrine 53(1): 7-17.
- Giri D, McKay V, Weber A, Blair JC (2015) Multiple endocrine neoplasia syndromes 1 and 2: Manifestations and management in childhood and adolescence. Arch Dis Child 100(10): 994-999.

Open Access Journals
- Aquaculture & Veterinary Science
- Chemistry & Chemical Sciences
- Clinical Sciences
- Engineering
- General Science
- Genetics & Molecular Biology
- Health Care & Nursing
- Immunology & Microbiology
- Materials Science
- Mathematics & Physics
- Medical Sciences
- Neurology & Psychiatry
- Oncology & Cancer Science
- Pharmaceutical Sciences