Connections between WarburgÃÆâÃâââ¬Ãâââ¢s and SzentgyorgyiÃÆâÃâââ¬Ãâââ¢s Approach about the Causes of Cancer
Szigeti GP, Szasz O and Hegyi G
DOI10.21767/2576-3903.100008
Szigeti GP1, Szasz O2 and Hegyi G3*
1Institute of Human Physiology and Clinical Experimental Research, Semmelweis University, Hungary
2Department of Biotechnics, St. Istvan University, Hungary
3Department of Complementary and Alternative Medicine, University of Pecs, Hungary
- *Corresponding Author:
- Gabriella Hegyi
Department of Complementary and Alternative Medicine
University of Pecs, Hungary
Tel: +36 30 922-5347
E-mail: drhegyi@hu.inter.net
Received date: November 30, 2016; Accepted date: December 26, 2016; Published date: January 20, 2017
Citation: Szigeti GP, Szasz O, Hegyi G. Connections Between Warburg’s and Szentgyorgyi’s Approach About Causes of Cancer. J Neoplasm. 2017, 1:2. doi: 10.21767/2576-3903.100008
Abstract
Numerous theories and hypotheses are published about the causes of cancer and its hallmarks. Two remarkable principles were established and debated for a long time. The first is the Warburg-effect, which based on mitochondrial dysfunction, connected to the intensive metabolic activity of the malignancy. The other is the Szentgyorgyi’s effect which describes the malignancy by changing of the cellular state (α ↔ β) explained by evolutional biology and supported by definitely improved dielectric properties of the malignant tissue from their normal counterpart while these theories have stable explanations, developed many controversies. Both are partial of completely revised time-by-time, showing new insights with new evidence as additions to these old ideas. Our objective is to demonstrate connections between these theories and start new considerations in the actual debates.
Keywords
Warburg-effect; Szentgyorgyi-effect; Fermentation; Irreversibility; Cancer
Introduction
Various theories and hypotheses are existing about the cause and origin of cancer from ancient medicine to a long line of new explanations. The advanced search for an answer was started more than a century ago with virus concept [1,2]; the genetic clues were later favoured [3,4], and the mutation concepts became popular [5-7]. Recently, the immunedependences [8], and connections with wound-repair have been intensively researched [9-13]. Despite the enormous efforts, the cause of cancer remains open [14], with groups trying to “fish” for the clue [15]. Despite even special quantum-physical explanation [16], the recent studies do not give a final solution [17,18]. The final explanation is missing, but the hallmarks are well defined [19,20]. More studies turn to the environmental, diet and habit origins of malignant diseases [21-23].
Most widely, cancer is believed to be an abnormal tissue triggered by a gene mutation. However, the proto-oncogene and the oncogene appear not only in cancerous cases [24], but with pregnancy [25], with embryogenesis [26,27], with the healing of wounds [28] and with the synthesis of growth factors [29]. Oncogenes show a great variety of anti-apoptotic functions with the cells taking part in the wound-healing [30]. To summarize, some oncogenic features appear in normal, non-malignant cases, such as in growth and repair processes. More cancer risk factors are associated with wound-like conditions or chronic stresses and poisoning. For instance, smoking cause’s chronic bronchitis and the risk factor can be found with lung cancer, hepatitis and so on [31-34]. Nobel laureate Albert Szentgyorgyi clearly described these environmental points [35]: It is unimportant that the monkey goes through the jungle; the important fact is that the jungle goes through the monkey, in the form of nutrition, water, and oxygen.
The appearance of the oncogene activity and the antiapoptotic functions is one of the most striking similarities between cancerous and certain standard processes, for example, growth, and repair. Since apoptosis is strongly connected with the mitochondrion, just like the oxidative ATP production, it can be suspected that functions of mitochondria also degrade temporarily with the growth and repair processes. The mode of the ATP production is also the starting point of the century-old Warburg theory [36].
Warburg started his work with the energetics of malignant growth. The cancerous tissue needs more energy than its healthy counterpart, because of the permanent fission and necessary amount of ATP for all steps to create daughter cells. The difference between the energetics of the healthy and malignant cells is that in malignancy, massive anaerobic (fermentative) ATP production takes place in the cytosol, while the aerobic process dominates in healthy cells. The cytosol could produce two ATPs from a glucose molecule in very simple steps, while the mitochondria produce 34 ATPs by the complicated and time-consuming citrate cycle process. The transport processes are also crucial: the function of the glucose, sodium, pyruvate and lactate, as well as the hydrogen ion transporters, have to serve the actual energy-production processes in time. These transports are also simpler in the case of anaerobic compared to aerobic energizing procedures. Note that this type of fermentative ATP production was characteristic at the beginning of the development of life on Earth. The early environment in our Globe lacked oxygen but was presumably rich in geochemically-produced molecules; producing ATP may have similarities to present-day forms of fermentation.
Method
Warburg’s effect
Warburg explained definite metabolic differences between the processes of malignant and healthy cells by the anaerobic glycolysis Warburg. The anaerobic glycolysis is a more intensive and simpler process to produce ATPs than the complicated Kerbs-cycle via the mitochondria [37]. Warburg stated the primary cause of cancer is a kind of mitochondrial dysfunction. He meant that the glycolytic fermentation of production of ATP energy molecules in the cells plays a decisional part. The discovery of the accelerated glycolysis to produce the ATP molecules was honored by Nobel-prize for Warburg.
The theory was soon induced debate. The question is immediate: is the shortage of the ATP due to the underperforming mitochondria, so the cause of the cancer is mitochondrial dysfunction or only a huge demand of ATP. The unusually high request for ATP is much more than the actively functioning mitochondria could provide for limited time applications via anaerobic glycolysis. It is similar to the massive sports activities when the aerobic process has a shortage of energy supply, so anaerobic glycolysis is used temporarily when the extreme load persists. This last supposes the permanent activity of mitochondria, and the fermentative APT production is the only addition to that. Is it the case in the malignant cell as well.
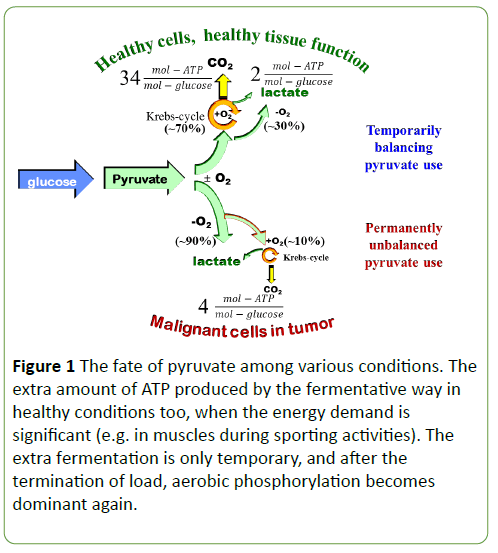
Not everybody was convinced by cancer being caused by damaged mitochondria [38,39]. It was found that cancer cells are also able to oxidize glucose comparable to those of healthy cells [40]. The argument reversed Warburg’s claim: cancer cells have reduced mitochondrial activity due to the demand of increased glycolytic flux, [41,42]. The relationship between the opinions is similar to the apparent controversy of the chickenand- egg “paradox” [43]; whether mitochondrial dysfunction decides the anaerobic glycolysis or reverse is valid: this fermentative way suppresses mitochondrial functions. Both models are supported by relevant data. The “enigma” of the primacy of one of the processes is unsolved [44]. The fate of pyruvate after the intake of glucose is the important process and not the glucose utilization alone. The activity of pyruvate kinase M2 is decisional. The reduced activity of this process prefers the anaerobic phosphorylation, while the high activity goes towards aerobic way [45]. The mitochondrial citric-cycle hindered by anaerobic glycolysis, it redirects pyruvate to ineffective but chemically pure and indeed quick ATP production. Warburg theory describes the lactate production as dominant, regardless of the available oxygen. Normal, healthy proliferative tissues have such processes too when the mitochondria are functional at full capacity for oxidative phosphorylation, but the increased demand is fulfilled by an addition of simple glycolysis, (Figure 1) [45]. The reprogramming process of the metabolic activity can be measured on mitochondrial DNA somatic mutation [46].
Figure 1: The fate of pyruvate among various conditions. The extra amount of ATP produced by the fermentative way in healthy conditions too, when the energy demand is significant (e.g. in muscles during sporting activities). The extra fermentation is only temporary, and after the termination of load, aerobic phosphorylation becomes dominant again.
The primary cause of cancer is anaerobic glycolysis stated by Warburg, and the mutation of the genome is a consequence of fermentative metabolism. His theory emphases the mitochondrial dysfunction in the cause of cancer, supposing that the malignant transformation produced by hypoxia.
The similarities of oncogenes’ activity and the anti-apoptotic functions in cancer and various healthy processes (like growth and reparation) are one of the most challenging facts in the present research. Due to the apoptotic processes as well as the oxidative ATP production, which are suppressed in numerous growth and reparative processes, the function of mitochondria degrades (at least temporarily). The observed degradation of mitochondria is the reason for the renaissance of Warburg’s theory, despite its controversial observations. The mitochondrial connection of metabolism of tumours is intensively investigated [47,48]. Warburg’s idea has since been revised [49,50] having a renaissance nowadays [51], and “returns in a New Theory of Cancer” [52], and new hypotheses are born on this basis [53,54].
Questions arise about the hereditary of dysfunctionality: is it stored in every mitochondrion, or the environmental conditions causing their dysfunction. The inherited dysfunction assumes the problem with the mitochondrion, while in the environmental cause with the cytosol and its compartments (mainly the cytoskeleton and ER) of the host cell.
If a cancerous cell separates then, other cancerous cells will be created. Does it mean that dysfunctionality is hereditary. Or are the environmental conditions hereditary.
The existence of mitochondria is common in most of the eukaryotes [55]. Some mitochondria, as well as its location, vary by cell type. The bean-like structure of mitochondria is not autonomic, this forms dynamic network in most cells, arranging a complex 3D branching with the cellular cytoskeleton. Mitochondria are distributed along microtubules, and their distribution is also correlated with the endoplasmic reticulum [56]. The complex formation with cytoskeleton regulates mitochondrial shape, and consequently, it can affect the function too [57]. Different structures of the mitochondrial network may manage a variety of physical, chemical and signalling processes governing advantages or disadvantages in the cellular function [58]. A single mitochondrion is often found in unicellular organisms mostly single mitochondrion serves the ATP production, but in the cells of multicellular systems, numerous mitochondria help the cellular functions. A huge number of mitochondria exist in human liver cells (approx. 1000-2000), filling up 20% of the entire cell volume and cooperatively working to serve the hepatocellular ATP demand [59]. Even the otherwise similar cells may vary substantially of their mitochondrial content, together with their size and membrane potential, [60]. The variation arises from uneven partitioning at cell division, producing extrinsic differences in energy demand and supplied cellular processes [61]. Interestingly, nestled mitochondria can be found between myofibrils of the muscle [56].
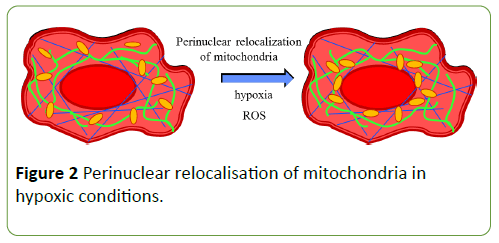
Mitochondria respond to fundamental stresses, including hypoxia, by changing their sub-cellular localization. The definite hypoxic conditions ignite mitochondrial fragmentation with spatial dynamics, and form perinuclear clustering [62,63]. Mitochondria relocate around the nuclei (Figure 2) [64].
The hypoxia-induced nuclear relocation of mitochondria is associated with increased nuclear reactive oxygen species (ROS) by the mitochondrial gene mutations, which can suppress the electron flux and so increases the mitochondrial ROS production [65]. This process targets gene (VEGF) expression, suggesting that mitochondrial clustering may play a role in allowing the ROS signal more directly to the nucleus.
In the phase of growing and reparation, the growth and repair genes (GR genes: the oncogenes and the protooncogenes… etc.) activate to create cells of the healthy network, which will become tissues [25,66,67]. When, for example, there is a wound and then healing starts: a platelet closes the injured capillaries. T-cells, macrophages, and NKcells migrate to the injured tissue to remove the debris and the injured cells. The T-cells, the monocytes and the macrophages present growth- and repair factors [68-70]. The GR genes activate the neighboring cells (bystander effect), which also give out GR factors. The GR factors require stem cells from the adjacent tissue and from the bone marrow to the edge of the wound [71]. The healing is perfect when the GR genes turn off or when the tumor suppressors turn on [72] and homeostasis is re-established. In tumorous cases, this process never finishes; the wound healing remains permanent [73].
Szentgyorgyi’s effect
Warburg recognized the excessive lactate generation of cancer cells. This phenomenon is the simple unicellular development. In this context, it looks like the evolutional return to simpler life, and so cancer could be regarded as “dismantling of multicellularity” [74]. This was the starting point of Szentgyorgyi is an aetiological approach: the disappearance of cellular collectivity [35]. He studied the decisional role of the pyruvate metabolic pathways in processes producing ATP. From this point of view, he distinguished two different states of the cells. When the fermentative processes are dominant in a cell, he denoted it with the alpha (α)-state. Development of the life started when the aggressive electron acceptor, the free oxygen, was not present, [75]. This is a simple, primitive form of life could exist at the beginning of evolution and the existing unicellular objects characterized this period. The character of this state is only replication, simple operating the living features; developing of a complex network and process division was not possible in this reproduction-oriented state. These cells are competing for their individual demands; acting autonomously, no cooperative communication is developed between the cells in α-state.
The presence of free oxygen in the evolution process changed the game of life. Cooperation of the individual cells was promoted, a new state, the beta (β)-state of life was developed. The presence of oxygen produced by the higher value of electric charges, made possible to form unsaturated proteins, which allowed more complex interactions. The diversity of life had been started. The cells in β-state are cooperatively connected, dividing the various tasks to optimize the intake of nutrients, the adaptability of the environment and the reproduction processes. The only multiplication in α- state became a complex diversity for optimal adjustment to life, optimizing the energy consumption of the life-processes. In this is a phase of evolution, avoid the poisoning direct oxygen exposure mitochondria was integrated into the cell. Mitochondria became the “power plant” of the cell, making oxidative ATP production with a complicated cycle but in a massive number of these energizing molecules.
Szentgyorgyi [35] described the life evolution on a cellular level with the interplay of α- and β-stages. The life of complexly organized living organisms based on their cells in β- state. The cell-division of this organism integrity became controlled. Due to the cooperative intercellular forces are slack in fission, there are inherent autonomic actions motivate the cellular division. Its control is mandatory to keep the system functional during the production daughter cells when a part of the structure should be dissolved and rearranged. The cellular status in the division is α-state, which locally terminates the cooperative complexity.
The α-state is the minimal conditional status of life when the cell completely concentrates on its fate. The complexity of life could easily be transformed into this basic state when the network is broken; the system becomes unstable, parallel with the forming of independent stable cells in α-state [76]. The cells in β-state are cooperative, energizing themselves with oxidative metabolism provided by their mitochondria. Their division should be strictly controlled by the networking cells and dynamic processes.
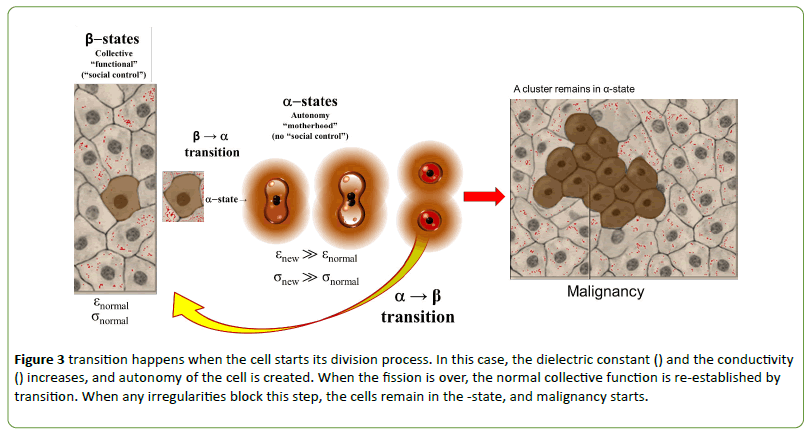
Normal cellular division (transforming locally the β-state to α-state) could be regular and irregular, depending on the reason which forcing it. Replacing the elder cells with young daughter cells, maintaining the cellular homeostasis is a regular process renewing the system working optimal, changing the weak-points of the network. When the cellular division is forced, constrained (e.g. reparations, woundhealing, even the embryonic development, etc.), the network tries to re-establish the homeostatic situation, regulating the processes with negative feedback control. Than α-state of cells form again, cells in β-state try (at least partly) devoted to producing the missing cells from the network, giving up their collective behavior. The remaining part of the collective network stabilizes the cooperative state and fixes the newborn cells from their α-state to β-state (Figure 3). The stability is broken by fission of the individual cell and again it turns into the α-state.
Figure 3: transition happens when the cell starts its division process. In this case, the dielectric constant () and the conductivity () increases, and autonomy of the cell is created. When the fission is over, the normal collective function is re-established by transition. When any irregularities block this step, the cells remain in the -state, and malignancy starts.
Discussion
The local circulatory disturbance causes permanent oxygen deficiency, which activates the anaerobic ATP production of the involved cells. This low-efficacy production should be intensive enough to energize the higher demand of the neoplasm. This increased transport intensity requests larger active surface area at the plasma membrane. Enlarging the active glycolytic transport could happen by broken celljunctions, increasing the effective glycolytic surface of the membrane by free surfaces. This process blocks the natural collective behaviour of the cells. The high metabolism of the forming cancerous cluster forces the neighbouring cells intensify their metabolic demand, (bystander effect) due to the redirected glucose transport from the healthy to the neoplasms. The anaerobe environment starts to be permanent and enlarging dynamically. The lost junctional connections of the cells create compulsory proliferation like Szentgyorgyi described it with the α-state. This is a typical precancerous state of the cluster of the involved cells.
In general, the higher energy-transport to an organ by sports activity or by other temporary high load of the actual function does not mean uncontrollable higher energy consumption of the cells involved. The limit is the actual energy distribution of the healthy transport network. The secondary network (cellular connections) is responsible for the collective organization of the cells in the organ. Consequently, the blockage of this secondary network blocks the collective behaviour too. The cancerous cells have mostly autonomic character, and the consequence of this is their proliferative behaviour.
The increase of the transport network is simple due to the growing mass of the organ. The neo-angiogenesis formally satisfies the demand of the growth, which could be explained by allometric evolution [77].
Due to the permanent hypoxic conditions, the blood tries to buffer the situation (electrolyte homeostasis), its pH grows and could deliver more oxygen than before. However, this extra oxygen probably supports the cells at the peripheries of the neoplasm, supplying the accelerated metabolism of the area. The enhanced metabolic activity is a direct mechanism of the growth of a neoplasm. The process will be soon anaerobic, which according to the Occam-razor [78], could dominate the metabolism by its simplicity compared to the more complicated Krebs-cycle.
Transport properties
The control of the balance of β-and α-states acts probably via the electromagnetic route [79]. The process to start division and complete it by networking to the neighbors has extreme energy needs. The transport of electrons in the internal signal pathway of protons in most of the transports. The electron is transferable in a chemical way; however, a fast hydrogen transmitter with low dissipation is necessary. This process is allowed by the structured water in the living state [80], which is semi-crystalline [81].
Mitochondria are shielded against the direct oxygen flux by the host cell. Its proper function supposes than an effective proton transporter which transports the hydrogen from the mitochondria to a more oxygen-rich part of the cell. The effective proton transport is driven by the active proton pumps energized by ATP (produced by mitochondria) as well. The proton alone does not exist in aqua-solution, it associates with a water molecule and could be transported only by a slow diffusion to the proton pumps. Having quick, effective proton transporter with small energy dissipation is necessary, which is simply the ordered water [78], which could transport the proton with high speed.
The monomer water molecule has a simple tetrahedron structure, which is slightly asymmetric by the two proton occupied positions and two lone pairs. However, the simple water has a rather complex structure in the bulk conditions [82,83]. The stochastic proton migration in hydrogen bonds makes the bulky water collective, [81]. The solid water (ice) has hydrogen-bridge connections over the entire volume [84]. The entire bridge bonded ice turns to water by the first kind of phase transition, but only a fraction of the bridges broke, about every seventh, which was approximated from the evaporation and melting heat ratio (We≈2256 kJ/kg and Wm≈334 kJ/kg). Remarkably, a high number of hydrogen bridges exist even at the boiling point of water [85]. In fact, the water is always a mixture of two phases [86,87]: disordered, highly dynamic, mainly monomer and ordered, clustered ones (the clusters could even have various structures, including an entirely closed clathrate with icosahedral symmetry [88]. The statistical, stochastic transformations of the phases make the water complex. The simple bifurcative phenomenon (the proton-chare is oscillating between the two possible positions) of the hydrogen bridges. The charge transfer of such oscillating bonds could be very different depending on the dwelling in the different states (the oscillation in general chemical bonding could be multi-state, not necessarily bifurcation only). The generalized solution of the bifurcative phenomena in living materials was worked out earlier, [77]. The ordered water was supposed to account for as much as 50% of the total amount of water in the living bodies [89], but the systematic investigations showed more of it [90,91].
Rearranging the water structure requires energy [92]. The energy intake took for the change of the structure, like the way, when water boils, the energy is massively supplied into the system, but the temperature is unchanged, fixed in 100°C. This drastic change (phase transition) in the structure of water modifies its physical properties (e.g. its dielectric constant) without changing its composition. It is a microscopic reordering in the water.
The hydrogen ion can be transported by hydrogen bridges. This high-speed and low dissipation of the transport propagation is based on Grotthuss-mechanism [93,94], where the proton can tunnel (jump) from one water cluster to another bridged by hydrogen bonds. The lifetime of H3O+ (hydronium ion) is rather small (~3·10-12s) so the speed of proton transport by Grotthuss-mechanism is approximately ten times higher than that by diffusion.
The Grotthuss-mechanism is, in fact, the propagation of ionizing of a water molecule. The dissociation and recombination steps are altered in “traveling”. The recombination-dissipation is a quantum-mechanical process; in principle, it is free of dissipation [95]. However, it has temperature dependence, and the vector potential is also able to modify the quantum states of the water [96,97], which could modify the chain processes.
As a consequence of malignant changes, the metabolism gradually favours the fermentation method (host cell performs the task instead of the blocked mitochondria). The metabolism is completed in the aqua-based electrolyte, and so its input and end-products are ions. In one side the molecules are involved in the oxidative cycle, like 6CO2+6H2O ↔ 12H+ + 6CO32-, while fermentation happens like 2CH3CHOHCOOH↔ 2CH3CHOHCOO- + 2H+. Equal proton production ensures the inert electric state of the solution, which fulfilled by more intensive fermentation and at the end the negative ions differ, the rapid growth of complex lactate-ion concentration increases its osmotic pressure. The unchanged pressure during the reaction increases the dissolvent monomer water, solving the ionic products in non-ordered water. Various malignancies show the change of water clusters to disordered monomers [98-100]. Consequently, the concentration of ordered water in cancerous tissues is smaller than in their healthy counterpart. This process weakens the networking signal between the species and between the cells. The hydrogen ions have reduced activity due to the weaker ionic transmitter. The intracellular pH decreases together with the weakened proton gradient in mitochondria, suppressing the efficacy of ATP production. The membrane potential of mitochondria grows trying to compensate the lowered proton-gradient. The higher potential of mitochondrial membrane lowers its permeability, decreases the mitochondrial permeability transition (MPT).
The process decreases the apoptotic availability where MPT has a crucial role, [101,102] Indeed, cancerous cells have high mitochondrial membrane potential and low K-channel expression [103]. The developed resistance to apoptosis supports the ATP production by the fermentative way to supply enough energy to the cell. Production of extra HSP chaperone stress proteins induced by the increased free ion concentration in the cytoplasm. This process also needs an extra amount of ATP. The growing HSP protection is antiapoptotic, and on this way, a complete block of apoptosis could be developed.
The processes are interconnected because the increase of the fissionability lowers the membrane potential, which again decreases the order of the water structure. The developing disorder raises the dielectric permeability (εr) of the water [35]. The higher εr effectively decreases the cell-cell adhesion, creating a positive feedback loop causing the cell-division and the uncontrolled proliferation [35]. It was detected as one of the signs of the tumor by NMR. The observation is also proof for Szentgyorgyi’s bioelectric considerations [104,105] of cancer development.
The primary effect of the malignant deviations connected to the changed metabolic pathways. The balance of the phosphorylation and fermentative metabolism determines the balance of the cellular collectively and cellular autonomy. The cooperative or non-cooperative conditions balanced by the energy exchange and signal transport between the cell and its actual environment. The variation of the metabolic pathway changes the intracellular transport too. Increased amount of heat is liberated by intensive energy flux of the fermentative metabolism, which builds up a temperature gradient between the extra- and intracellular electrolytes. The growing temperature could reach a critical threshold of the heat flow, turning the heat-flow from conductive to convective [106], which is the phenomenon of the Bernard instability [107]. The ionic flows through the cellular membrane promoted by the convective heat flow, which increases the intake of glucose. This again supports the fermentative metabolism and changes the intracellular circulations [108,109]. The mitochondrial oxidative metabolism is down-regulated by these complex processes.
The energy loss in optimal biochemical ATP production is large. The glucose liberates 2881 kJ/mol in the phosphorylation (aerobic) process, but the produced ATP is only 1159 kJ/mol; the efficacy of the process is ≈ 40%. The “missing” value (1722 kJ/mol) wasted by the non-used chemical species and by the heat, which makes a body temperature independent from its environment.
From an energetic point of view, high-intensity massproduction of ATP is necessary to fulfill the strong energy demand of the permanent proliferation of malignant cells. The higher metabolic rate could be routinely measured by positron emission tomography (PET) [110]. The reaction rate of the simple fermentative reaction could be 100 times quicker (approximated from the positron annihilation data) [111] than the oxidative way. However, the oxidative way has an at least 18 times higher efficacy due to the ATP amount at the end.
The result is funny: the simple, primitive fermentative process produces at the end six times more ATP during a given period than its high efficacy, but there was too complicated an oxidative counterpart, which is why the extreme situations constrain the anaerobe metabolism. The balance is broken at out of normal homeostasis situation. The glucose demand in fermentation at a given time is higher by two orders of magnitude than in oxidative phosphorylation. The high glucose demand needs intensive glucose transport, as well as the endproduct (lactate) also has to be transported away. The low efficacy of fermentation produces more heat in the lesion, and the locally higher temperature promotes the diffusion processes, as well as inducing higher blood perfusion and easier permeability of the vessel walls.
The massive and speedy demand for energy (in fact glucose), should be supported by the transport availabilities. The intracellular transport has numerous restrictions of ATP energy-transfer [112] also; the diffusion has very complex heterogeneity in the cytosol, as described by Levy’s flight [113]. The anomalous diffusion in the cytosol needs support from the cytoskeleton, [114,115] which in the case of proliferative conditions partly of completely collapsed [116]. Consequently, the demand for the massive energy supply from mitochondria has numerous limitations, and in fact favours the simple and extra speedy anaerobic glycolysis, which is directly produced in the place where it is used, in the cytosol. A further challenge for the transport is the collapse of the waterhydrogen- bridge network by the lowered membrane potential of malignant cells. The missing Grotthuss mechanism, in this case, limits the diffusivity of protons, and the disordered electrolyte limits the diffusion of any ions [117].
The picture, however, is not as simple as it looks. Many malignant tumors have massive aerobic, oxidative phosphorylation in mitochondria, together with more or less anaerobic ATP production as well. This apparent controversy may be explained by differences in tumor size, hypoxia, and the sequence of oncogenes activated [118]. This picture has a similarity to sport medicine, when the prompt energy supply to the muscles in the sprint-sports are solved by the anaerobic fermentation [119]. However, when the extra need is over, the normal aerobic function is re-established. In tumors, however, the load and the consequent need for ATP is never terminated; instead, it exponentially grows in time.
Generalisation
The simple comparison could be made between Szentgyorgyi’s α- and β-states and Warburg’s metabolic pathways approach. The electron transport and change of electronic properties of the tissues supposed by Szentgyorgyi in malignant transformation [35,120,121], and the mitochondrial dysfunction proposed by Warburg [36,37], have a common origin. The oxygen-deficiency and in consequence the actual energy flow are in the center of both considerations. Szentgyorgyi’s theory α-states well corresponds with the dominance of the fermentative metabolism in Warburg’s theory, while the normal phosphorylation of mitochondria shows common with β-states. The α-state prefers intensive anaerobic ATP production, while β-state works with perfect mitochondrial function.
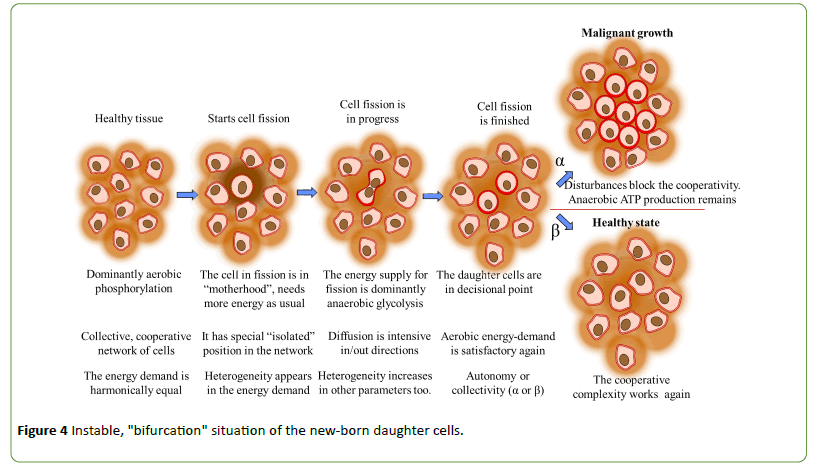
Cells in any stage work in both the possible glucose utilization, only the dominance of activities defines their state. The balance of the ATP production could be described by the cell status of cooperativity (α ↔ β); or by the metabolic process (anaerobic ↔ aerobic). Both formulae define what is the acting the part of metabolic energizing (host-cell ↔ mitochondrion). The meaning of both theories expresses the actual energetic state of the cell. The energy flux and cooperability are tightly interrelated, the growing energy-flux weakens the cellular cooperativity, makes the arrangement more individual, autonomic. Oppositely, the low energy flux creates cooperative cells which are forming complex, sophisticated networks, and they are highly effective in their energy production and their adaptation to changing environmental conditions (Figure 4).
The concentration of the components determines the amount of dissolvent in the aqueous electrolytes of living objects, promoted by the order-disorder transitions of the cytoplasm as well as depend on the osmotic water flow through the cell membrane. The outside stimuli inside enrichment of a component could produce a misbalance of ionic concentrations, which could be a constraint or a normal aging process too.
The decisional order-disorder transformation of water changes the diffusion of the hydrogen ions, which mirrored in the dielectric constant of the medium [77]. The disorder increases the dielectric constant of the aqueous solution, increasing the ability of electrical isolation. On this way, the promoted charge-division and the suppressed polymerization activity creates positive feedback to the fermentation processes. The actual balance of ATP production is broken, the fermentation became important; the cell turns to α-state. Usually, it is not a malignant transition, which happens with any regular cell fission too. Fermentative ATP production characterizes the "motherhood" of the cell in the division, energizing the "delivery" of the daughter cells. The "individualism" of the mother-cell is due to its extremely increased energy demand during the fission process. However, after the creation of the daughter cells, the previous healthy order should be re-established. The motherhood is in the "infancy" of the evolution, having autonomy instead of common networking. Their "infancy" at the start is standard like the "babyhood" is normal after deliveries. However, it is not normal when it is too long. The “babyhood” must be transferred back to collective order after finishing the fission. When the daughter cells do not find the way back to the cooperability, the cells are “frozen” in the uncontrolled proliferative α-state. The frozen state is a defect of the complex controlling mechanism [35], which correlates with the single “renegade cell” concept [106], Weinberg describes cancer as a long process to produce "a renegade cell" as an ancestor of the billion-cell group called cancer. Epidemiological research showed the complexity of the developing of cancer. It is demonstrated on a statistical basis that at least five different coincident mutations are involved in the development of malignancy [67].
One of the driving forces to re-establish the β-state after the division of the cell is the reduced demand of energy-flux. During the cell-fission, tremendous energy should supply the process, which spends to produce many proteins and other cellular elements (lipids, enzymes, etc.). The conditions of α-state fit for this demand. When the fission finishes, the energy consumption lowers but remains doubled compared to the single original mother cell. The dissolvent capacity is enough supplying the new cells. The overall loss of the cellular energy demand acts like cooling phase transition from liquid to solid, (disorder-order transition). Similar phase change happens in this case as when the division was started, the only difference is the opposite direction of the process. The proton bifurcation in hydrogen-bridges reorganizes the order without opposing driving force; even the neighboring environment supports this arranging process.
The new-born cells are more negatively charged than the host cells. This negative state vanishes by the gathering of the elder cells in the collective network. After the cellular division, the daughter cells separated. The larger overall membrane surfaces of the separated volumes (double cells) limit the intracellular dynamics and restructure it for the final cytoskeletal form. The increased surface area down-regulates the heat-flow through the cellular membrane and turns the energy-exchange back from convective to the conductive [66]. The intensive diffusion of the large-molecule like glucose is not supported ever more in the rearranged conductive situation, so the slow but massive mitochondrial ATP production became regular, the normal homeostatic conditions will dominate again.
The repair mechanism could be blocked or limited. An obvious reason could be the permanent irritation by a mechanical, chemical or physical (e.g. ionization) factor. However, in the case of any damage, some cells produce such a functional state which repairs the actual dysfunction. The growth- or repairing-phase special genes are activated to produce such cells, which repair the damaged tissue [69,68]. Growth and reparative factors are released [71,72]. Protooncogenes became active in the area [122], collecting stem cells to the wound [73], which are repaired by their differentiation [123]. After the process, all of the activation genes are down-regulated, or tumor suppressors are activated [124], and the normal homeostasis became re-established. In the cancerous state, the repair conditions are not blocked after finishing the reparation process itself. Permanent reparation demands depletion of the available stem cells [125,126], and will be more emphasized the third way to repair: the protooncogenes are activated, and malignant transformation could happen to induce clinical cancer [127]. The malignant transformation of the wound makes secretion of protooncogenes [128-130], and intensive capture and stimulation of the stem cells from other places [131,132], the cancer cells produce repair [133,134], molecules increasing the stem-cell concentration in the cancer tissue [135].
When an island is formed from the cells in the α-state in healthy tissue, then current starts between the island and the tissue inducing cell migration and proliferation. This current is called the injury current [136]. The process is essential in wound healing [137], and the current is gradually limited by the healing wound. In the case of a cluster of cells in the α- state, it has positive feedback, increased by time, the grouping of the new-born cells, having no possibility to neutralize themselves to the potential level of the normal surroundings. This process never heals this “wound,” even the opposite, it growths. This process has extra high energy demand, so the massive ATP production of the fermentative way is preferred and became irreversible in this stage.
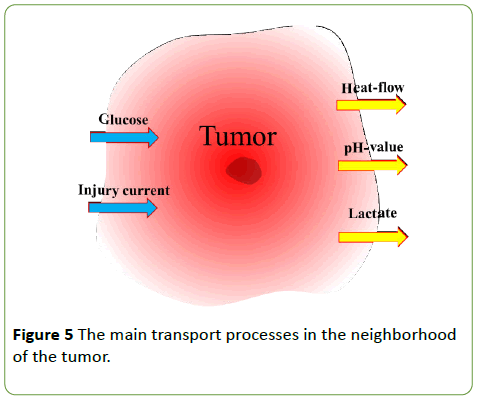
The boundary of the cancerous tissue embedded in its healthy neighborhood is not sharp and not well defined. The smeared border of the cancerous tissue is also the boundary of the different biological, chemical and electric processes, and is the interface of special transports (Figure 5). The glucose influx and lactate efflux are due to fermentation, which causes low pH in the volume; while the injury current is due to charge inhomogeneity. The high intensity of low efficacy fermentation creates a higher temperature in the tumor than its neighborhood, inducing heat-flow from the tumor.
Despite the huge variety of tumors and healthy tissues, the allometric scaling (biasing) [138] could be discussed in a universal frame, [139], even on the subcellular energy consumption as well as even the mitochondria and the respiratory complexes [138]. Naturally, these differences are not based on independent properties, their connections and interactions are important governors over the process of the expansion of tumor volume, their complexity controls the dynamism and actual state of the malignancy. The interplay of the conditions of ATP production and the polarization ability of the cells are well connected with each other and with the pattern forming of the developing structures [140,141]. The method of ATP production influences the ionic concentration and pH in the extracellular electrolyte, which naturally affects the polarization properties of the media and interacts with the decreased membrane potential of malignant cells while the conductivity of the volume will also be modified. The diverging scaling is connected to the high metabolic rate where the scaling exponent approaches the unity which induces high vascularisation and intensive cellular proliferation at the frontiers of the different tissues. Due to the more negative average charge of the malignant tissue, a definite fluctuation of the electric field strength is also developed on the boundaries of the tumor.
The scaling exponents could follow changes in the metabolic processes, [142]. The lowest average scaling exponent shows the surface-volume ratio of ÃÆâÃâââ¬Â¦Ãâââ¬Â. The dominantly anaerobic metabolism of benthic invertebrates (n=215) have exponent pmean=0.63 (near to ÃÆâÃâââ¬Â¦Ãâââ¬Â), [143]. However the animals (n=496) having dominantly mitochondrial ATP production have and exponent pmean=0.74, (near to ¾), [140]. Moreover, the scaling of the metabolic activity is different in mitochondrial or non-mitochondrial metabolism. The normal mitochondrial metabolism performs the Krebs cycle, and its scaling exponent is well distributed around p=¾, [77,137], while the scaling exponent of fermentative respiration is near to ÃÆâÃâââ¬Â¦Ãâââ¬Â [144]. Remarkably, the healthy mammalian cells and the mitochondria that are 5 orders of magnitude lighter, as the respiratory complexes (having further 5 orders of magnitude less mass than mitochondria) are fit well on the allometric scaling with ¾, [145].
A further interesting relation between energy flux and cooperatively is that, in vitro, the specific metabolic rate in the cultured cells (overall metabolic rate normalized on the actual mass) is constant [146] no scaling could be introduced. In vitro the practically infinite nutrients from the media electrolyte keep the cells autonomic; they are in α-state.
Conclusion
Warburg’s effect fits well with Szentgyorgyi’s theory. The dominance of anaerobic glycolysis in malignant cells characteristic for the autonomous α-state, while the proper mitochondrial function is connected to the collective β-states. Central consideration of both principles is the extra energy demand, which appears by quick and simple nonmitochondrial ATP production and in consequences the autonomy of the cells using this process. The phenomenon is like the increased ATP supply of the heavy load of muscles in some sports; however, that fermentative step is reversible, the ATP production will be mitochondrial when the energy-demand turns back to normal. This increased ATP production is not reversible in malignancy; the proliferative cells remain in autonomy (α-state). In this case, the lack of negative feedback (lowering the energy consumption) is missing. The huge variety of disturbances either genetic or environmental origin blocks the reversibility, the demand for extra energy growths by continued proliferation of cells.
References
- Rous P (1910) A transmissible avian neoplasm (Sarcoma of the common fowl). J Exp Med 12: 696-705.
- Weiss RA, Vogt PK (2011) 100 years of Rous sarcoma virus. J Exp Med 208: 2351-2355.
- Boveri T (1929) ZurFrage der Entstehung Maligner Tumoren (Gustav Fischer, Jena); English translation: The Origin of Malignant Tumours by M Boveri(ed). Williams and Wilkins,Baltimore.
- Knudson AG (2001) Two genetic hits (more or less) to cancer. Nat Rev Cancer 1: 157-162.
- Wunderlich V (2007) Early references to the mutational origin of cancer. Int J Epidemiol36: 246-247.
- Nordling CO (1953) A new theory on the cancer-inducing mechanism. Br J Cancer 7: 68-72.
- Burnet FM (1957)Cancer a biological approach. Br Med J 1: 841-847.
- Dunn GP, Bruce AT, Ikeda H, Old LJ, Schreiber RD (2002) Cancer immunoediting: From immunosurveillance to tumour escape. Nature Immunology 3: 991-998.
- Dvorak HF (1986) Tumours: Wounds that do not heal. Similarities between tumour stroma generation and wound healing. N Engl J Med. 315: 1650-1659.
- Tennant RW (1993) A perspective on non-mutagenic mechanisms in carcinogenesis. Environ Health Perspect 101: 231-236.
- Sonnenschein C, Soto AM (2000) Somatic mutation theory of carcinogenesis: why it should be dropped and replaced. MolCarcinog 29: 205-211.
- Feinberg AP, Ohlsson R, Henikoff S (2006) The epigenetic progenitor origin of human cancer. Nat Rev Genet 7: 21-33.
- Oleg VB, Denis MS (2015) Pathogenesis of cancer: cancer reparative trap: J Cancer Ther 6: 399-412.
- Dimitrios T, Frederick PL, David JH (1996) What causes cancer? Scientific American 80-87.
- Merlino G, Khanna C (2007) Fishing for the origins of cancer. Genes Dev 21: 1275-1279.
- Hameroff SR (2004) A new theory of the origin of cancer: quantum coherent entanglement, centrioles, mitosis, and differentiation. Biosystems 77: 119-136.
- Rycaj K, Tang DG (2015) Cell-of-origin of cancer versus cancer stem cells: assays and interpretations. Cancer Res 75: 4003-4011.
- Andrew CW, William EL (2015) Refining the role for adult stem cells as cancer cells of origin. Trends in Cell B 25: 11-20.
- Hanahan D, Weinberg RA (2000) The hallmarks of cancer. Cell 100: 57-70.
- Hanahan D, Weinberg RA (2011) Hallmarks of Cancer: The Next Generation; Cell 144: 646-674.
- Gold LS, AmesBN, Slone TH(2002) Misconceptions about the causes of cancer. In: D Paustenbach (ed) Human and environmental risk assessment: Theory and practice. New York, John Wiley & Sons, pp. 1415-1460.
- Danaei G, Vander Hoom S, Lopez AD, Murray CJ, Ezzati M (2005) Causes of cancer in the world: Comparative risk assessment of nine behavioural and environmental risk factors. Lancet 366: 1784-1793.
- Clapp RW, Jacobs MM, Loechler EL (2008) Environmental and occupational causes of cancer: New evidence 2005-2007. Rev Environ Health 23: 1-37.
- Anderson MW, Reynolds SH, You M, Maronpot RM (1992) Role of proto-oncogene activation in carcinogenesis. Environ Health Perspect 98: 13-24.
- MedenH, Marx D, Fattahi A, Rath W, Kron M, et al. (1994)Elevated serum levels of a C-ERBB-2 oncogene product in ovarian cancer patients and in pregnancy. J Cancer Res ClinOncol 120: 378-381.
- Attie-Bitach T, Abitbol M, Gérard M, Delezoide AL, Auge J,et al. (1998) Expression of the RET proto-oncogene in human embryos. Am J Med Genet 80: 481-486.
- Quenby SM, Gazvani MR, Brazeau C, Neilson J, Lewis JDI, et al. (1999) Oncogenes and tumour suppressor genes in first trimester human foetal gonadal development. Mol Hum Reprod 5: 737-741.
- Okada Y, Saika S, Hashizume N, Kobata S, Yamanaka O, et al. (1996) Expression of fos family and jun family proto-oncogenes during corneal epithelial wound healing. Curr Eye Res 15: 824-832.
- Stiles CD (1985) The biological role of oncogenes–insights from platelet-derived growth factor: Rhoads Memorial Award lecture. Cancer Res 45: 5215-5218.
- Friess H, Lu Z, Graber HU, Zimmermann A, Adler G, et al. (1998) Bax, but not bcl-2, influences the prognosis of human pancreatic cancer. Gut 43: 414-421.
- Emerich S, Sohn OS, Wang CX, Seibert E, Tsurutani J, et al. (2005) Induction of preneoplastic lung lesions in guinea pigs by cigarette smoke inhalation and their exacerbation by high dietary levels of vitamins C and E. Carcinogenesis 26: 605-612.
- Platz EA, De Marzo AM (2004) Epidemiology of inflammation and prostate cancer. J Urol 1712: 36–40.
- Murthy NS, Mathew A (2009) Risk factors for pre-cancerous lesions of the cervix. Eur J Cancer Prev 9: 5–14.
- Molloy RM, Sonnenberg A (1997) Relation between gastric cancer and previous peptic ulcer disease. Gut 40 : 247–252.
- Szentgyorgyi A (1978) The living state and cancer. Marcel Dekker Inc. New York.
- Warburg O (1956) On the origin of cancer cells. Science 123: 309-314.
- Warburg O (1996) Oxygen: The creator of differentiation.Biochemical Energetics. Academic Press, New York In: The Prime Cause and Prevention of Cancer. Revised lecture at the meeting of the Nobel-Laureates on June 30, Lindau, Lake Constance, Germany.
- Weinhouse S (1976) The Warburg hypothesis fifty years later. Cancer Res ClinOncol 87: 115-126.
- Weinhouse S (1956) On respiratory impairment in cancer cells. Science 124 : 267-269.
- Weinhouse S, Millington RH, Wenner CE. (1951) Metabolism of neoplastic tissue. I. The oxidation of carbohydrate and fatty acids in transplanted tumours. Cancer Res 11: 845-850.
- Sussman I, Erecinska M, Wilson DF (1980) Regulation of cellular energy metabolism: The Crabtree effect. BiochimBiophysActa 591: 209-223.
- Crabtree HG (1992) Observations on the carbohydrate metabolism of tumours. Biochem J23: 536-545.
- Senyilmaz D, Teleman AA (2015) Chicken or the egg: Warburg effect and mitochondrial dysfunction; F1000. Prime Reports 7: 41.
- Kim HH, Joo H, Kim T, Kim E, Park SJ, et al. (2009) The mitochondrial Warburg effect: A cancer enigma.Interdisciplinary Bio Central 1: 1-7.
- Matthew G,HeidenV, Thompson CB (2009) Understanding the Warburg effect: The metabolic requirements of cell proliferation. Science 324: 1029.
- Kim AY (2014) Mitochondrial DNA somatic mutation in cancer. Toxicol Res 30: 235-242.
- Semenza GL (2008) Tumor metabolism: Cancer cells give and take lactate. The Journal of Clinical Investigation 118: 3835-3837.
- Rathmell JC, Newgard CB (2009) Biochemistry. A glucose-to-gene link. Science 324 : 1021-1022.
- Schulz TJ, Thierbach R, Voigt A, Drewes G, Mietzner B, et al. (2006) Induction of oxidative metabolism by mitochondrial frataxin inhibits cancer growth. The Journal of Biological Chemistry 281 : 977–981.
- Miles KA, Williams RE (2008) Warburg revisited: Imaging tumour blood flow and metabolism. Cancer Imaging 8: 81-86.
- Heiden MGV, Cantley LC, Thompson CB (2009) Understanding the Warburg effect: The metabolic requirements of cell proliferation Science 324 : 1029-1033.
- Garber K (2004) Energy boost: The Warburg effect returns in a new theory of cancer. Journal Nat CancInst 96: 1805-1806.
- Seyfried TN, Mukherjee P (2005) Targeting energy metabolism in brain cancer: review and hypothesis. Nutrition & Metabolism 2: 30-38.
- Xiaolong M, Riordan NH (2006) Cancer is a functional repair tissue. Medical Hypotheses 66: 486-490.
- Roger AJ, Svärd SG, TovarJ, ClarkCG, Smith MW,et al.(1998) A mitochondrial-like chaperonin 60 gene inGiardia lamblia: Evidence that diplomonads once harboured an endosymbiont related to the progenitor of mitochondria. Proceedings of the National Academy of Sciences (National Academy of Sciences) 95 (1): 229–234.
- Soltys BJ, Gupta RS (1992) Interrelationships of endoplasmic reticulum, mitochondria, intermediate filaments, and microtubules—A quadruple fluorescence labelling study. Biochem Cell Biol 70: 1174-1186.
- Rappaport L, Oliviero P, Samuel JL (1998) Cytoskeleton and mitochondrial morphology and function. Mol and Cell Biochem 184: 101-105.
- Hoitzing H, Johnston IG, Jones NS (2015) What is the function of mitochondrial networks? A theoretical assessment of hypotheses and proposal for future research. Bioessays 37: 687–700.
- Alberts B, Johnson A, Lewis J, Raff M, Roberts K, et al. (1994) Molecular biology of the cell. New York: Garland Publishing Inc.
- Das Neves RP, Jones NS, Andreu L, Gupta R, Enver T, et al. (2010) Connecting variability in global transcription rate to mitochondrial variability. PLOS Biology 8 (12): e1000560.
- Johnston IG, Gaal B, Das Neves RP, Enver T, Iborra FJ, et al. (2012) Mitochondrial variability as a source of extrinsic cellular noise. PLOS Computational Biology 8: e1002416.
- Tracy K, Dibling BC, Spike BT, Knabb JR, Schumacker P, et al. (2007)BNIP3 is aRB/E2Ftarget gene required for hypoxia-induced autophagy. Mol Cell Biol 27: 6229–6942.
- Al-Mehdi AB, Pastukh VM, Swiger BM, Reed DJ, Patel MR, et al. (2012) Perinuclear mitochondrial clustering creates antioxidant-rich nuclear domain required for hypoxia-induced transcription. SciSignal5: ra47
- Boland ML, Chourasia AH, Macleod KF (2013) Mitochondrial dysfunction in cancer. Frontiers in Oncology 3: 1-28.
- Wallace DC (2005) Mitochondria and Cancer: Warburg Addressed; Cold Spring Harbour Symposia on Quantitative Biology 70: 636-649.
- Caubet JF, Bernaudin JF(1988). Expression of the c-fos proto-oncogene in bone, cartilage and tooth forming tissues during mouse development. Biol Cell;64(1): 101-104.
- Carrasco D (1994) Developmental expression of the mouse c-rel proto-oncogene in hematopoietic organs. Development 120: 2991-3004.
- Blotnick S, Peoples GE, Freeman MR, Eberlein TJ, Klagsbrun M (1994)T-lymphocytes synthesise and export heparin-binding epidermal growth factor-like growth factor and basic fibroblast growth factor, mitogens for vascular cells and fibroblasts: Differential production and release by CD4+ and CD8+ T cells. PNAS 91: 2890-2894.
- Workalemahu G (2003) Human CD-T lymphocytes express and synthesise connective tissue growth Factor: Effect of IL-15 and TGF-ß1 and comparison with aß-T lymphocytes. J Immunol 170: 153-157.
- Soslau G, Morgan DA, Jaffe JS, Brodsky I, Wang Y (1997)Cytokine mRNA expression in human platelets and a megakaryocytic cell line and cytokine modulation of platelet function. Cytokine 9(6): 405-411.
- Son Y, Hong H, Kim J (2004) Identification of substance – p as an early inductive cytokine of corneal wound and its possible role in the mobilisation of mesenchymal stem cell and corneal wound healing. Invest Ophthalmol Vis Sci 45: 1423.
- Noszczyk BH, Majewski ST (2001) p63 expression during normal cutaneous wound healing in humans. PlastReconstrSurg 108(5): 1242-1247.
- Meng X, Riordan NH (2006) Cancer is a functional repair tissue. Medical Hypotheses 66: 486-490.
- Alfarouk KO, Shayoub MEA, Muddathir AK, Elhassan GO, Bashir AHH (2011) Evolution of tumour metabolism might reflect carcinogenesis as a reverse evolution process (Dismantling of multicellularity). Cancers 3: 3002-3017.
- Szentgyorgyi A (1960) Introduction to a Submolecular Biology. Academic Press: New York, London.
- Szasz A (1991) An electrically driven instability: The living-state (Does the room temperature superconductivity exist?). PhysiolChemPhys Med NMR 23: 43-50.
- West GB, Woodruf WH, Born JH (2002) Allometric scaling of metabolic rate from molecules and mitochondria to cells and mammals. ProcNatlAcadSci USA 99: 2473-2478.
- Gauch HG (2003) Scientific method in practice, Cambridge University Press,UK.
- Szentgyorgyi A (1998) Electronic Biology and Cancer. Marcel Dekker, New York.
- Szasz A, D Van Noort, Scheller A, Douwes F (1994) Water states in living systems. I. Structural aspects. PhysiolChem Phys. 26(4): 299-322.
- Cope FW (1969) Nuclear magnetic resonance evidence using D2O for structured water in muscle and brain. Biophys J 9: 303-319.
- Eisenberg D, Kauzmann W (1969) Structure and properties of water. Clarendon Press, Oxford, England.
- Nelson GM, Enderby JE (1985) Water and aqueous solutions. Adam Hilger, Bristol-Boston.
- Maryan M, Szasz A, Szendro P, Kikineshy A (2005) Synergetic model of the formation of non-crystalline structures. Journal of Non-Crystalline Solids 351: 189-193.
- Nemethy G, Scheraga HA (1962) Structure of water and hydrophobic bonding in proteins. I. A model for the thermodynamic properties of liquid water. J ChemPhys 36: 3382-3400.
- Marjan M, Kurik M, Kikineshy A (1999) Two structure model of liquid water. Modelling Simul Mater SciEng 7: 321-331.
- Urquidi J (2001) Theoretical studies on liquid water. PhD Thesis Texas Tech University. Texas.
- Pauling L (1959) The structure of water. In: Hadzi D, Thompson H (eds) Hydrogen bonding, Pergamon Press Ltd: London.
- Cope FW (1975) A review of the applications of solid state physics concepts to biological systems. J Biol Phys. 3: 1-41.
- Hazlewood CF, Nichols BL, Chamberlain NF (1969) Evidence for the existence of a minimum of two phases of ordered water in skeletal muscle. Nature 222: 747–750.
- Hazlewood CF, Chang DC, Medina D (1972) Distinction between the Pre-neoplastic and neoplastic state of murine mammary glands. ProcNatlAcadSci USA 69: 1478-1480.
- Chidanbaram R, Ramanadham M (1991) Hydrogen bonding in biological molecules-an update. Physica B 174: 300-305.
- Agmon N (1995) The Grotthuss mechanism. ChemPhysLett 244: 456-462.
- Markovitch O, Agmon N (2007) Structure and energetics of the hydronium hydration shells. J PhysChem A: 111(23): 2253-2256.
- Maryan MI, Kikineshy A, Szendro P (2001) Modelling of the dissipative structure of water. ActaTechnologicaAgriculturaeSlovacaUniversitasAgriculturaeNitriae 3: 77-80.
- Szendro P, Koltay J, Szasz A, Vincze GY (1999) Is the structure of the water convertible in physical way? Hungarian Agricultural Engineering 12: 43-45.
- Fu-MingT (2003) Solvent effects of individual water molecules. In: Buch, V., Devilin, J.P. (eds) Water in Confining Geometries, Cluster Physics. Springer Verlag, Berlin.
- Gniadecka M, Nielsen OF, Wulf HC (2003) Water content and structure in malignant and benign skin tumours. Journal of Molecular Structure 661-662: 405-410.
- Beall PT (1979) Water-relaxation times of normal, pre-neoplastic, and malignant primary cell cultures of mouse mammary gland. In: 23rd Annual Meeting of the Biophysical Society, Atlanta, Georgia, USA,p: 26-28.
- Chung SH, Cerussi AE, Klifa C (2008) In vivo water state measurements in breast cancer using broadband diffuse optical spectroscopy. Phys Med Biol 53: 6713-6727.
- Fiskum G (2000) Mitochondrial participation in ischemic and traumatic neural cell death. Journal of Neurotrauma 17: 843-855.
- Ichas F, Mazat JP (1998) From calcium signalling to cell death: two conformations for the mitochondrial permeability transition pore. Switching from low- to high- conductance state. Biochimica et BiophysicaActa 1366: 33-50.
- Bonnet S, Archer SL, Allalunis-Turner J, Haromy A, Beaulieu C, et al. (2007) A Mitochondria-K+ channel axis is suppressed in cancer and its normalisation promotes apoptosis and inhibits cancer growth. Cancer Cell 11: 37-51.
- Damadian R (1971) Tumour detection by nuclear magnetic resonance. Science 171: 1151-1153.
- Szentgyorgyi A (1968) Bioelectronics: A study on cellular regulations. Defence and Cancer. Acad Press,New York.
- Kurakin A (2009) Scale-free flow of life: on the biology, economics, and physics of the cell. TheorBiol Med Model 6: 6
- Getling AV, Rayleigh-Benard C (1998) Structures and dynamics. World Scientifics, Singapore.
- Hochachka PW (1999) The metabolic implications of intracellular circulation. ProcNatlAcadSci USA 96: 12233-12239.
- Coulson RA (1986) Metabolic rate and the flow theory: a study in chemical engineering. Comp BiochemPhysiol A Comp Physiol 84: 217-229.
- Oehr P, Biersack HJ, Coleman RE (eds) (2004) PET and PET-CT in Oncology. Springer Verlag: Berlin-Heidelberg.
- Larson SM (2004) Positron emission tomography-based molecular imaging in human cancer: exploring the link between hypoxia and accelerated glucose metabolism. Clin Cancer Res 10: 2203-2204.
- Sepp M, Vendelin M, Vija H, Birkedal R (2010) ADP compartmentalisation analysis reveals coupling between pyruvate kinase and ATPases in heart muscle; Biophysical Journal 98: 2785–2793.
- Dross N, Spriet C, Zwerger M, Müller G, Waldeck W, et al. (2009) Mapping eGFP oligomer mobility in living cell nuclei. PLoS ONE 4: e5041.
- Brangwynne CP, Koenderink GH, MacKintosh FC, Weitz DA (2009) Intracellular transport by active diffusion. Trends in Cell Biology 19: 423-428.
- Regner BM, Vucinic D, Domnisoru C, Bartol TM, Hetzer MW, et al. (2013) Anomalous diffusion of single particles in cytoplasm. Biophysical Journal 104: 652–1660.
- Friedman E, Verderame M, Winawer S, Pollack R (1984) Actin cytoskeletal organisation loss in the benign-to-malignant tumour transition in cultured human colonic epithelial cells, Cancer Research, 44: 3040-3050.
- Podolsky RJ (1958) Transport processes in electrolyte solutions. Am ChemSoc 80: 4442-4451.
- Jose C, Bellance N, RossignoIR (2011) Choosing between glycolysis and oxidative phosphorylation: A tumour's dilemma; Biochimica et BiophysicaActa 1807: 552–561.
- Bogdanis GC (2012) Effects of physical activity and inactivity on muscle fatigue. Frontiers in Physiology 3: 142-152.
- Szentgyorgyi A. (1941) Towards a new biochemistry? Science 93 (2426): 609–611.
- Szentgyorgyi A (1946) Internal photo-electric effect and band spectra in proteins. Nature 157: 875–875.
- Weinberg RA (1998) One renegade cell. Basic Books, A Member of the Perseus Books Group, New York.
- Cairns J (1975) The Cancer Problem. Scientific American 233(5): 64-72.
- Lavon N, Yanuka O, Benvenisty N (2004) Differentiation and isolation of hepatic-like cells from human embryonic stem cells. Differentiation 72(5): 230-238.
- Shin BJ, Ching SS (2003) A case of limbal stem cell deficiency in a patient with chronic mucocutaneouscandididiasis. Invest Ophthalmol Vis Sci 44: 1359.
- Houghton J, Stoicov C, Nomura S, Rogers AB, Carlson J (2004) Gastric cancer originating from bone marrow-derived cells. Science 306: 1568-1571.
- Ouahes N, Phillips TJ, Park HY (1998) Expression of c-fos and c-Ha-rasprotooncogenes is induced in human chronic wounds. DerrnatolSurg 24(12): 1354-1357.
- Huang S, Trujillo JM, Chakrabarty S (1992) Proliferation of human colon cancer cells: role of epidermal growth factor and transforming growth factor. Int J Cancer 52: 978-986.
- Dahiya R, Lee C, Haughney PC, Chui R, Ho R, et al. (1996) Differential gene expression of transforming growth factors alpha and beta, epidermal growth factor, keratinocyte growth factor, and their receptors in foetal and adult human prostatic tissues and cancer cell lines. Urology 48: 963-970.
- Mizuno K, Sone S, Orino E, Nii A, Ogura T (1994) Autonomous expressions of cytokine genes by human lung cancer cells and their paracrine regulation. Jpn J Cancer Res 85: 179-186.
- Zhang H, Vutskits L, Pepper MS (2003) VEGF is a chemoattractant for FGF-2-stimulated neural progenitors. J Cell Biol 163: 1375-1384.
- Cicuttini FM, Begley CG, Boyd AW (1992) The effect of recombinant stem cell factor (SCF) on purified CD34-positive human umbilical cord blood progenitor cells. Growth Factors 6: 31-39.
- Meyer NA, Muller MJ, Herndon DN (1994) Nutrient support of the healing wound. New Horiz 2: 202-214.
- Printz C (2001) Spontaneous regression of melanoma may offer insight into cancer immunology. J Natl Cancer lnst 93: 1047-1048.
- Turner AM, Zsebo KM, Martin F, JacobsenFW, BennettLG(1992) Non-haematopoietic tumour cell lines express stem cell factor and display c-kit receptors. Blood 80(2): 374-381.
- Rosch PJ, Markov MS (2004) Bioelectromagnetic medicine. Marcell Decker Inc: New York.
- Reid B, McCaig CD, Zhao M, SongB (2005) Wound healing in rat cornea: The role of electric currents. FASEB J 19: 379-386.
- Calder III WA (1984) Size, Function and life history. Dover Publications Inc. Mineola: New York.
- West GB, Born JH (2000) Scaling in biology. Oxford University Press, USA.
- Deering W, West BJ (1992) Fractal physiology. IEEE Eng Med Biol 11: 40-46.
- West BJ (1990) Fractal physiology and chaos in medicine. World Scientific, Singapore.
- Moses ME, Hou C, Woodruff WH, West GB, Nekola JC, et al. (2008) Revisiting a model of oncogenic growth: estimating model parameters from theory and data. Am Nat 171: 632-645.
- Pamatmat MM (2005) Measuring aerobic and anaerobic metabolism of benthic infauna under natural conditions. Journal of Experimental Zoology 228: 405-413.
- West GB, Brown JH (2005) The origin of allometric scaling laws in biology from genomes to ecosystems: towards a quantitative unifying theory of biological structure and organisation. Journal of Experimental Biology 208: 1575-1592.
- Lane N (2006) Mitochondria: Key to Complexity. In: Martin W (ed) Origins of Mitochondria and Hydrogenosomes, Chapter 2, Springer, Heidelberg, Germany.
- Brown MF, Gratton TP, Stuart JA (2007) Metabolic rate does not scale with body mass in cultured mammalian cells. Am J PhysiolRegulIntegr CompPhysiol 292: R2115-R2121.

Open Access Journals
- Aquaculture & Veterinary Science
- Chemistry & Chemical Sciences
- Clinical Sciences
- Engineering
- General Science
- Genetics & Molecular Biology
- Health Care & Nursing
- Immunology & Microbiology
- Materials Science
- Mathematics & Physics
- Medical Sciences
- Neurology & Psychiatry
- Oncology & Cancer Science
- Pharmaceutical Sciences