The Role of BRCA1 and BRCA2 Genes in the Appearance of Pediatric and Adolescent Disorders
Ioannis Drikos, Alexandros Sachinidis, Ioanna Vassi4, Effrossyni Boutou
DOI10.21767/2576-3903.100015
Ioannis Drikos1,2*, Alexandros Sachinidis3, Ioanna Vassi4 and Effrossyni Boutou5
1Pediatric Clinic, General Hospital of Elefsina, Thiassio, Greece – Hellas, Gennimatas Avenue Magoula, 19600, Greece
2Department of Neonatology, National and Kapodistrian University of Athens, Greece –Hellas, Greece
3Second Propaedeutic Department of Internal Medicine, Medical School, Aristotle University of Thessaloniki, Hippocration Hospital, Thessaloniki, Greece – Hellas, Greece
4Department of Philoshophy, Neurolinguistics in Education, National Kapodistrian University of Athens, Greece
5Molecular Genetics Laboratory, Thalassaemia and Hemoglobinopathies Center, Laiko General Hospital, Athens, Greece – Hellas, Greece
- *Corresponding Author:
- Dr. Ioannis Drikos
Pediatric Clinic, General Hospital of Elefsina, Thiassio, Greece - Hellas, Gennimatas Avenue Magoula, 19600, Greece
Tel: 2105563292
E-mail: johndrikos@yahoo.com
Received date: June 29, 2017; Accepted date: July 20, 2017; Published date: July 30, 2017
Citation: Drikos I, Sachinidis A, Vassi I, Boutou E (2017) The Role of BRCA1 and BRCA2 Genes in the Appearance of Pediatric and Adolescent Disorders. J Neoplasm. Vol.2 No.2:6. doi: 10.21767/2576-903.100015
Abstract
The genes BRCA1 and BRCA2 appear crucial role in development of hereditary breast and ovarian cancer. Hundreds of different types of mutations have been identified in these genes. The high risk mutations inactivate a very important and foolproof DNA repair process, thus increasing considerably the risk of diseases development such as breast and ovarian cancer.
These genes seem to have a significant influence and participation in appearance of pediatric diseases other than breast and ovarian cancer being important molecules in DNA repair process and cell cycle progression. Therefore further exploration and evaluation of these genes in the appearance of pediatric diseases may enhance the importance of prenatal audit.
Keywords
BRCA1; BRCA2; Pediatric and adolescent disorders; Cell cycle control
Abbreviations
AML: Acute Myelogenic Leukemia; BRCA1: Breast Cancer Gene 1; BRCA2: Breast Cancer Gene 1; CNS: Central Nervous System; DSB: Double Strand Brakes; FA: Fanconi Anemia; FANCD: Fanconi Anemia Disease; HR: Homologous Recombination; NHL: Non-Hodgkin’s Lymphomas; NTDS: Neural Tube Defects; RB: Retinoblastoma; SBMM: Spina Bifida Meningo Myelocele; WRN: Werner Syndrome; WT: Wilms Tumor
Introduction
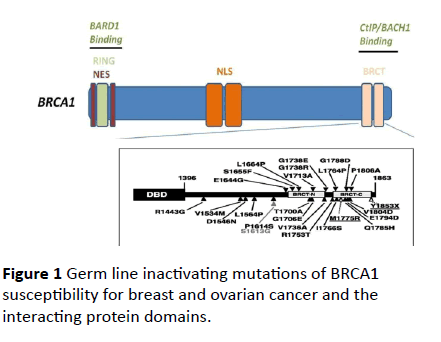
The BRCA1 and BRCA2 genes have a crucial role in multiple organ systems. BRCA1 and BRCA2 are key molecules of cell cycle control and participate in development of several pediatric diseases such as anemia Fanconi, spina bifida, meningomyelocele, tumors of CNS fetal origin, premature mortality and infantile epileptic encephalopathy. The appearance of heterozygosite in BRCA1 or BRCA2 genes predisposes to cancer in childhood (Figure 1). Therefore the identification of mutations of BRCA1 and BRCA2 in the general population and the availability of screening tests becomes important for early diagnosis and evaluation. The purpose of this review is to provide pediatric diseases related to mutations in BRCA1 or BRCA2 genes displaying the main role of these genes in several diseases except breast and ovarian cancer.
Literature Review
The involvement of the BRCA2 gene in CNS tumors with fetal origin
Nowadays genetic analysis has been made to identify the BRCA genes mutations. These methods based on sequencing analysis of exonic and adjacent intronic regions of the BRCA1 and BRCA2 genes by quantitative procedures of polymerase chain reaction. The identified germline mutations such as 6174abIT and 886delGT of BRCA2 gene were identified in children with CNS embryonic tumors and confirmed that every parent was a carrier of ΒRCA2 mutations [1,2]. Mutations in BRCA1 and BRCA2 genes are prevalent in Ashkenazi Jewish populations [1,2]. In many cases, the presence of these tumors was directly related to Fanconi anemia even if in 17 studied families by Reid et al. the diagnosis of Fanconi anemia was absent. Mutations of genes BRCA1 and BRCA2 have already been determined in many types of tumors, such as Wilms tumors, brain tumors including medulloblastoma, multiple erythema, astrocytoma, acute myeloid leukemia and acute lymphoblastic leukemia. In these cases where identified 23 allelic mutations of the BRCA2 gene. Among these twenty-one patients developed childhood cancer and 9 of them developed brain tumors [2]. In assays of genetic material were shown that in five cases of analyzed brain tissues had the mutation 886delGT of BRCA2 gene [2], while according to Alter et al. 27 published cases of FANCD1 and mutants of gene BRCA2 revealed correlation between the deletion 6174delGT of BRCA2 and brain tumor development [3].
Identification of mutations of BRCA1 gene in children with spina bifida and meningomyelocele (NTD)
The neural tube defects (NTDs) include a major group of genetic disorders with a total incidence of about 1-2 individuals per 1000 live births in the United States [4]. While NTD which characterized by loss of closure of the neural tube in the head is fatal, the appearance of spina bifida in the spinal cord area (spina bifida meningomyelocele) could be treated successfully. Spina bifida (meningomyelocele) (SBMM) is an important clinical entity that is caused by the limited closure of the spinal canal [5,6]. In mices deficient for BRCA1 gene 40% of the embryos are presented with spina bifida and anencephaly [6]. Moreover, in embryos deficient for BRCA1 gene the differentiation of neural tube is disorganized while people are characterized by increased risk of cell death [6].
A recent study by Wang et al. showed that the interaction between BRCA1 and Gadd45a is essential for normal embryonic development in mouse models and the deregulation of the BRCA1 gene by lack of exon 11 can induce apoptosis in mouse embryos [7]. In mouse models, the expression of the BRCA1 (Bard1) is higher in the crust compared to the cerebellum and hippocampus [8]. In a mouse model with a deficit of exon 10 of BRCA1 the 40% of fetuses detected with exencephaly with disorganized neuroepithelium even if the spina bifida was normal in mices with BRCA1 mutations [6,8]. According to Terri et al. two microsatellite regions are associated with the low number of repetitions of genes D17S1323 and D17S1322 at CEPH cells and the BRCA1 gene is associated with the occurrence of spina bifida suggesting that BRCA1 gene is essential for neuronal development [9].
The involvement of BRCA1 gene in patients with premature mortality
According to the literature mutations of BRCA1 gene have already determined in a neonate with fatal neonatal rigidity and multifocal seizure syndrome. Neonates with such gene mutations have hypertension, persistent seizures, frequent episodes of apnea, microcephaly, stagnation in the development of the head, lack of any development progress [10,11] and most patients survived until 21 months. DNA sequencing analysis data identified heterozygous variations in BRCA1 gene. The exact role of BRAT1 has not yet clarified, but there are evidences from in vitro studies in which the reading frame is affected. This results revealed differential interaction of the cell-cycle proteins [BRCA1 and ATM] [8,12], disruption of the intracellular localization Drikos et al. [13] and possibly alterations in mitochondrial function [8].
BRCA1 and infantile epileptic encephalopathy
The BRCA1 gene is mutated in a neonatal with fatal infantile epileptic encephalopathy. Recently described three cases of infants with persistent myoclonic seizures, hypertension and convulsions, poor growth of the head, inability to swallow, periods of apnea and bradycardia which led to cardiac arrest and death. In all cases, identified a mutation in the BRCA1 gene (BRAT1). In addition the study of Hanes et al. presented two homozygotes with mutation of BRCA1 gene [14]. In 2012, the Puffenberger et al. described a case of convulsion which started from the mold, small head circumference at birth, axial stiffness, rigidity of limbs, minor language disabilities and lack of developmental progress. Also noted frequent manifestations of spontaneous apnea and bradycardia leading to cardiopulmonary dysfunction. Subjects identified a novel homozygous mutation of c.638_639insA of BRCA1 gene associated with severe corticobasal decomposition [15,16]. Therefore speculated that the instability and dysfunction of BRCA1 protein is responsible for the occurrence of catastrophic epilepsy and neuronal atrophy [15,16]. Additionally two pups from Japan revealed progressive cerebral degeneration atrophy of the cerebellum and delayed myelination of white brain tissue [15,16].
Mutations in the genes BRCA1 and BRCA2 and their correlation with the occurrence of retinoblastoma
Genetic analysis of the BRCA1 correlates this gene with methylation procedures of luminal tumors. The genomic instability is therefore common in cells that are characterized by mutations in BRCA1 and BRCA2. Recently it has been shown that the tumor suppressor PTEN gene is disrupted by the BRCA1 gene rearrangements [17]. FISH techniques in the BRCA1 gene repealed rearrangements in cancer cells resulting in disruption of gene function and appearance of retinoblastoma [18]. An interesting observation is also recorded according to high frequencies of breakages of RB1 gene especially in cancer subtypes with mutations of BRCA1 gene and cells with methylation of BRCA1, indicating that the BRCA1 and its structural changes affects tumor progression [19]. The genetic association of BRCA1 and RB1 genes is further supported by the identification of the physical interaction [20]. Thus, the highly proliferative capacity of basal breast carcinomas with mutated BRCA1 cells could potentially be due to the interaction of BRCA1 and RB1. The presence of chromosomal deficits of RB1 genes are associated with the mutated forms of the BRCA1 and significantly determines reduced expression of pRB to those cell types [19,20].
Additionally intracellular pathway of RB1/E2F regulates the expression of FA genes [21]. In patients suffering by MYCN and retinoblastoma, without affecting the path RB1/E2F, the FANCA gene is downregulated in comparison to patients who have not disturbed intracellular pathway of RB1/E2F [5,22]. The hyperactivity of the FA/BRCA pathway associated with increased resistance to certain drugs whereas suppression of FA/BRCA associated with increased susceptibility [2,23].
The association of BRCA1 gene mutations in appearance Werner syndrome
The BRCA1 protein interacts directly with the helicase WRN which appears exonuclease activity and the interaction between WRN and BRCA1 increases in cell types which exposed to DNA mutagens. The treatment of DNA mutations in vivo in ICLs required the action of WRN as helicase but not as exonuclease. These observations suggest that the proteins WRN and BRCA1 are involved in DNA repair in cases of ICLs. The ICLs form a covalent attachment between the two strands of the DNA helix [24]. These interactions of WRN and BRCA1 during formation of DSBs in S phase may prevent the incorrect homologous recombination [HR] [25,26]. The absence of expression and activity of BRCA1 or WRN genes induce increased frequency of DSBs during phase S such as WRN being located in the region gH2AX foci as studied by Lan et al. The BRCA1 therefore support the helicase activity of the WRN as BRCA1 and WRN interact in repair DNA processes. In Holliday repair process formed in fork structure WRN is determined [25-27]. The BRCA1 support the function of WRN is fork structures [28].
Mutations of BRCA genes in patients with Wilms tumor
The Wilms tumor (WT) is a tumor with embryonic origin of the kidney which appears at 1 in 10,000 children. The incidence is determined in 1-3% of cases of childhood cancer and associated with mutations in WT1 and WT2 genes that have been mapped to chromosome 17q21 and 19q13.1-3. However, a significant percentage of cases the WT tumors appear to be related to these genetic regions [29]. Until recently there were restricted data correlate childhood cancers, such as WT with mutations in BRCA1 and BRCA2 genes. Patients who have undergone during childhood in chemotherapy and radiation approaches are more likely to spill malignancies such as cancer [30]. Henderson et al. reported an increased risk of secondary cancer during childhood in patients with bone sarcomas, renal tumors, Hodgkin's lymphoma and WT [31].
Cotton et al. determined that 17% of patients with tumors of childhood die after the appearance of secondary neoplasm defined as late effects of treatment [32]. Furthermore Henderson et al. identified an 11-fold increased risk of secondary cancer in patients receiving treatment with radiation, especially breast cancer probably because of mutations in BRCA1 and BRCA2 [31,32]. According to this we can say that breast cancer is a common secondary cancer in surviving patients with Wilms tumor (WT) [33].
Additionally Robison reported a significant association of breast cancer in patients with WT [34]. Cheng et al. identified 29 cases of invasive breast cancer among survivors with WT and 4 cases occurred before the age of 40 [35]. Apart from treatment procedure of irradiation genetic factors may also affect tumor incidence of breast cancer in survivors of WT such as familial mutation in the BRCA1 [36] or overexpression of IGF2 gene [37].
Fanconi anemia and mutations in BRCA1 and 2 genes
Fanconi anaimia is a prime disease of pediatric age which has been associated with mutations in BRCA1 and BRCA2 genes. Fanconi anemia (FA) is transmitted as an autosomal recessive inheritance disease with direct linkage on chromosome X characterized by multiple congenital defects, failure of bone marrow and increased risk of cancer. De novo mutations of the BRCA1 gene is closely associated with the occurrence of Acute myelogenic leukemia (AML) such as 32% of primary AML tumors and 75% of secondary AML tumors characterized by reduced expression of the BRCA1 gene [38]. Alongside BRCA2 mutations detected also in cases of non- Hodgkin's lymphomas (NHL) [38]. The protein products of the BRCA1 and BRCA2 genes interact with the protein of Fanconi anemia forming a powerful functional complex.
Discussion
The complex is capable of directing the Fanconi protein within the region of repair process. Mutations in the BRCA2 gene (FANCD1) may appear in early childhood [39]. Some epidemiological studies have shown an increased risk of leukemia/lymphoma, in patients with specific mutations in the genes BRCA1 or BRCA2 [40]. The proteins BRCA1, BRCA2 and Fanconi regulate the integrity of the human genome and operated via the FA/BRCA- pathway to repair DNA DSBs. The FA/BRCA pathway activated by the monoubiquitination of FANCD2 and FANCI, which is considered to be crucial for the activation procedure. The FA pathway has also been shown to be upregulated in infection such as HPV - infection [41] and BRCA1 appears to be localized to the cellular component of the centrosome.
According to this FANCA and BRCA1, interact with the transcription factor E2F3 and appears to affect the function of the centrosome [42]. In agreement with studies of uterine cancers revealed restricted expression of FANCA and BRCA1 genes [43] and these tumors exhibit dysfunction of centrosome [44]. The FANCD2-Ub can interact with PCNA proliferating cell nuclear antigen [34,44] and BRCA2/RAD51 functionally interacts with FANCD2 complex [45,46]. The BRCA2/RAD51 complex is not sufficient to promote the process of homologous recombination in the absence of the FANCD2-Ub and especially to localize complex into the nucleus after HU exposure [47]. Furthermore mutations may cause destabilization of BRCA2/FANCD1 complex influence homologous recombination demonstrating a nonfunctional complex in DSB sites.
Conclusion
BRCA1 and BRCA2, appear a very important role in DNA repair, homologous recombination and transcription. If there is damage to important control molecules of cell cycle, such as p53, RAD51, BRCA1 and BRCA2 disrupt proliferation and carcinogenesis. BRCA1 and BRCA2 apart from carcinogenesis seem to have a decisive role in the emergence of many pediatric diseases making prenatal screening important in determining the potential risk of such pediatric disorders.
Authors’ Contributions
Ioannis Drikos, Alexandros Sachinidis, Ioanna Vassi, Effrossyni Boutou participated in the design of the study and helped to draft the manuscript. All authors read and approved the final manuscript.
Declaration of Competing Interests
The authors declare that they have no competing interests.
Ethical Approval
This article does not contain any studies with human participants performed by any of the authors.
References
- Krainer M, Silva-Arrieta S, FitzGerald MG, Shimada A, Ishioka C, et al. (1997) Differential contributions of BRCA1 and BRCA2 to early-onset breast cancer. N Engl J Med 336: 1416–1421.
- Tischkowitz M, Ameziane N, Waisfisz Q, De Winter JP, Harris R, et al. (2003) Bi-allelic silencing of the Fanconi anaemia gene FANCF in acute myeloid leukaemia. Br J Haematol 123: 469–471.
- Alter BP, Rosenberg PS, Brody LC (2007) Clinical and molecular features associated with biallelic mutations in FANCD1/BRCA2. J Med Genet 44: 1–9.
- Northrup H, Volcik KA (2000) Spina bifida and other neural tube defects. Curr Probl Pediatr 30: 313–332.
- Ganguly A, Shields CL (2010) Differential gene expression profile of retinoblastoma compared to normal retina. Mol Vis 16: 1292–1303.
- Hunt GM, Oakeshott P (2003) Outcome in people with open spina bifida at age 35: prospective community based cohort study. BMJ Cancer 326: 1365–1366.
- Xu X, Qiao W, Linke SP, Cao L, Li WM, et al. (2001) Genetic interactions between tumor suppressors BRCA1 and p53 in apoptosis, cell cycle and tumorigenesis. Nat Genet. 28: 266–271.
- Ouchi M, Ouchi T (2010) Regulation of ATM/DNA-PKcs phosphorylation by BRCA1-associated BAAT1. Genes Cancer 1: 1211–1214.
- Tischkowitz MD, Chisholm J, Gaze M, Michalski A, Rosser EM (2004) Medulloblastoma as the first presentation of Fanconi anemia. J Pediatr Hematol Oncol 26: 52–55.
- Saitsu H, Yamashita S, Tanaka Y, Tsurusaki Y, Nakashima M, et al. (2014) Compound heterozygous BRAT1 mutations cause familial Ohtahara syndrome with hypertonia and microencephaly. J Hum Genet. 59: 687-690.
- Straussberg R, Ganelin-Cohen E, Goldberg-Stern H, Tzur S, Behar DM, et al. (2015) Lethal neonatal rigidity and multifocal seizure syndrome—Report of another family with a BRAT1 mutation. Eur J Paediatr Neurol 19: 240–242.
- Drikos I, Nounesis G, Vorgias C (2009) Characterization of cancer linked BRCA1-BRCT missense variants and interaction with phosphoptotein targets. Proteins Journal. 77: 464-476.
- Drikos I, Boutou E, Vorgias C (2009) BRCA1-BRCT cancer-related point mutations alter subcellular localization of BRCA1 in vitro. FEBS Journal. 276: 1-364.
- Hanes I, Kozenko M, Callen D (2015) Lethal neonatal rigidity and multifocal seizure syndrome--A misnamed disorder? Pediatr Neurol 53: 535-540.
- Saitsu H, Yamashita Y, Tanaka Y, Tsurusaki Y, Nakashima M, et al. (2014) Compound heterozygous BRAT1 mutations cause familial Ohtahara syndrome with hypertonia and microcephaly. J Hum Gen 59: 687–690.
- Hart AR, Sharma R, Rittey CD, Mordekar SR (2015) Neonatal hypertonia: A diagnostic challenge. Dev Med Child Neurol 57: 600-610.
- Saal LH, Gruvberger-Saal SK, Persson C, Leovgren K, Jumppanen M, et al. (2008) Recurrent gross mutations of the PTEN tumor suppressor gene in breast cancers with deficient DSB repair. Nat Genet 40: 102–107.
- Herschkowitz JI, He X, Fan C, Perou CM (2008) The functional loss of the retinoblastoma tumor suppressor is a common event in basal like and luminal B breast carcinomas. Breast Cancer Res 10: 75-87.
- Ciriello G, Cerami EG, Sander C, Schultz N (2012) Mutual exclusivity analysis identifies oncogenic network modules. Genome Res 22: 398–406.
- Ravasi T, Suzuki H, Cannistraci CV, Katayama S, Bajic VB, et al. (2010) An atlas of combinatorial transcriptional regulation in mouse and man. Cell 140: 744–752.
- Hoskins EE, Gunawardena RW, Habash KB, Wise-Draper TM, Jansen M, et al. (2008) Coordinate regulation of Fanconi anemia gene expression occurs through the Rb/E2F pathway. Oncogene 27: 4798–4808.
- Hoskins EE, Morreale RJ, Werner SP, Higginbotham JM, Laimins LA, et al. (2012) The fanconi anemia pathway limits human papillomavirus replication. J Virol 86: 8131–8138.
- Wreesmann VB, Estilo C, Eisele DW, Singh B, Wang SJ (2007) Downregulation of Fanconi anemia genes in sporadic head and neck squamous cell carcinoma. ORL J Otorhinolaryngol Relat Spec 69: 218–225.
- Thompson LH (2005) Unraveling the Fanconi anemia-DNA repair connection. Nature Genet 37: 921–922
- Prince PR, Emond MJ, Monnat RJ (2001) Loss of Werner syndrome protein function promotes aberrant mitotic recombination. Genes Dev 15: 933–938.
- Prince PR, Ogburn CE, Moser MJ, Emond MJ, Martin GM, et al. (1999) Cell fusion corrects the 4-nitroquinoline 1-oxide sensitivity of Werner syndrome fibroblast cell lines. Hum Genet 105: 132–138.
- Constantinou A, Tarsounas M, Karow JK, Brosh RM, Bohr VA, et al. (2000) Werner’s syndrome protein (WRN) migrates Holliday junctions and co-localizes with RPA upon replication arrest. EMBO Rep 1: 80–84.
- Cobb JA, Schleker T, Rojas V, Bjergbaek L, Tercero JA, et al. (2005) Replisome instability, fork collapse, and gross chromosomal rearrangements arise synergistically from Mec1 kinase and RecQ helicase mutations. Genes Dev. 19: 3055–3069.
- Rapley EA, Barfoot R, Bonaiti-Pellie C, Chompret A, Foulkes W, et al. (2000) Evidence for susceptibility genes to familial Wilms tumour in addition to WT1, FWT1 and FWT2. Br J Cancer 83:177–183.
- D’Andrea AD, Grompe M (2003) The Fanconi anaemia/BRCA pathway. Nat Rev Cancer 3: 23–34.
- Levitus M, Rooimans MA, Steltenpool J, Cool NF, Oostra AB, et al. (2004) Heterogeneity in Fanconi anemia: evidence for 2 new genetic subtypes. Blood 103: 2498–503.
- Meetei AR, De Winter JP, Medhurst AL, Wallisch M, Waisfisz Q, et al. (2003) A novel ubiquitin ligase is deficient in Fanconi anemia. Nat Genet 35: 165–170.
- Venkitaraman AR (2004) Tracing the network connecting BRCA and Fanconi anaemia proteins. Nat Rev Cancer 4: 266–276.
- Howlett NG, Taniguchi T, Olson S, Cox B, Waisfisz Q, et al. (2002) Biallelic inactivation of BRCA2 in Fanconi anemia. Science 297: 606–609.
- Cheng W, Kusumoto R, Opresko P, Sui X, Huang S, et al. (2006) Collaboration of Werner syndrome protein and BRCA1 in cellular responses to DNA interstrand cross-links. Nucleic Acids Research 34: 2751–2760.
- Mohaghegh P, Karow JK, Brosh JR, Bohr VA, Hickson ID (2001) The Bloom’s and Werner’s syndrome proteins are DNA structure-specific helicases. Nucleic Acids Res 29: 2843–2849.
- Poot M, Yom JS, Whang SH, Kato JT, Gollahon KA, et al. (2001) Werner syndrome cells are sensitive to DNA cross-linking drugs. FASEB J 15:1224–1226.
- Scardocci A, Guidi F, D'Alou F, Gumiero D, Fabiani E, et al. (2006) Reduced BRCA1 expression due to promoter hyermethylation in therapy-related acute myeloid leukaemia. Brit J Cancer 95: 1108-1113.
- Wagner J, Tolar J, Levran O, Scholl T, Deffenbaugh A, et al. (2004) Germline mutations in BRCA2: Shared susceptibility to breast cancer, early onset leukemia, and Fanconi anemia. Blood 103: 3226-3229.
- Hemminki K, Scelo G, Boffeta P, Mellemkjaer L, Tracey E, et al. (2005) Second primary malignancies in patients with male breast cancer. Brit J Cancer. 92: 1288-1292.
- Howlett NG, Taniguchi T, Olson S, Cox B, Waisfisz Q, et al. (2002) Biallelic inactivation of BRCA2 in Fanconi anemia. Science. 297: 606–609.
- Kim H, D’Andrea AD (2012) Regulation of DNA cross-link repair by the Fanconi anemia/BRCA pathway. Genes Dev 26:1393–1408.
- Cirilo PD, Marchi FA, De-Camargo Barros-Filho M, Rocha RM, Domingues MAC, et al. (2013) An integrative genomic and transcriptomic analysis reveals potential targets associated with cell proliferation in uterine leiomyomas. PLoS ONE 8: 57901-57912.
- Shan W, Akinfenwa PY, Savannah KB, Kolomeyevskaya N, Laucirica R, et al. (2012) A small-molecule inhibitor targeting the mitotic spindle checkpoint impairs the growth of uterine leiomyosarcoma. Clin Cancer Res 18: 3352–3365.
- Long DT, Räschle M, Joukov V, Walter JC (2011) Mechanism of RAD51-dependent DNA interstrand crosslink repair. Science 333: 84–87.
- Lan L, Nakajima S, Komatsu K, Nussenzweig A, Shimamoto A, et al. (2005) Accumulation of Werner protein at DNA double-strand breaks in human cells. J Cell Sci 118: 4153–4162.
- Naim V, Rosselli F (2009) The FANC pathway and BLM collaborate during mitosis to prevent micronucleation and chromosome abnormalities. Nat Cell Biol 11: 761–768.

Open Access Journals
- Aquaculture & Veterinary Science
- Chemistry & Chemical Sciences
- Clinical Sciences
- Engineering
- General Science
- Genetics & Molecular Biology
- Health Care & Nursing
- Immunology & Microbiology
- Materials Science
- Mathematics & Physics
- Medical Sciences
- Neurology & Psychiatry
- Oncology & Cancer Science
- Pharmaceutical Sciences